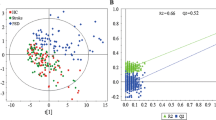
Abstract
A metabonomics study based on GC/MS and multivariate statistical analysis was performed involving 28 post stroke depressed (PSD) patients, 27 post-stroke non-depressed (PSND) patients and 33 healthy subjects to investigate the biochemical perturbation in their plasma samples. The outcome of this study showed that there was distinctive metabolic profile for PSD patients. Seven sentinel metabolites showed marked perturbations in PSD patients' blood. The introduction of metabonomics approach may provide a novel metabonomic insight about PSD and the sentinel metabolites for classifying PSD.


Similar content being viewed by others
References
Adams HP Jr., Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD (1999) Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of Org 10172 in acute stroke treatment (TOAST). Neurology 53:126–131
Arrieta-Cruz I, Su Y, Knight CM, Lam TK, Gutierrez-Juarez R (2013) Evidence for a role of proline and hypothalamic astrocytes in the regulation of glucose metabolism in rats. Diabetes 62:1152–1158
Arvelo MB, Cooper JT, Longo C, Daniel S, Grey ST, Mahiou J, Czismadia E, Abu-Jawdeh G, Ferran C (2002) A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology 35:535–543
Belgardt BF, Bruning JC (2010) CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 1212:97–113
Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817
Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TM (1995) Prevalence of depression after stroke: the perth community stroke study. Br J Psychiatry 166:320–327
Corthesy-Theulaz I, den Dunnen JT, Ferre P, Geurts JM, Muller M, van Belzen N, van Ommen B (2005) Nutrigenomics: the impact of biomics technology on nutrition research. Ann Nutr Metab 49:355–365
Coster J, McCauley R, Hall J (2004) Glutamine: metabolism and application in nutrition support. Asia Pac J Clin Nutr 13:25–31
Ding X, Yang S, Li W, Liu Y, Li Z, Zhang Y, Li L, Liu S (2014) The potential biomarker panels for identification of major depressive disorder (MDD) patients with and without early life stress (ELS) by metabonomic analysis. PLoS One 9:e97479
Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23:5928–5935
Feng B, Wu S, Lv S, Liu F, Chen H, Yan X, Li Y, Dong F, Wei L (2007) Metabolic profiling analysis of a D-galactosamine/lipopolysaccharide-induced mouse model of fulminant hepatic failure. J Proteome Res 6:2161–2167
Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L (2000) Metabolite profiling for plant functional genomics. Nat Biotechnol 18:1157–1161
First MB, Donovan S, Frances A (1996) Nosology of chronic mood disorders. Psychiatr Clin North Am 19:29–39
Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG (2007) Free amino acid and dipeptide changes in the body fluids from alzheimer’s disease subjects. Amino Acids 32:213–224
Gainotti G, Marra C (2002) Determinants and consequences of post-stroke depression. Curr Opin Neurol 15:85–89
Hagler L, Herman RH (1973) Oxalate metabolism. I. Am J Clin Nutr 26:758–765
Hawkins RA (2009) The blood-brain barrier and glutamate. Am J Clin Nutr 90:867S–874S
Hawkins RA, Simpson IA, Mokashi A, Vina JR (2006) Pyroglutamate stimulates Na + −dependent glutamate transport across the blood-brain barrier. FEBS Lett 580:4382–4386
Kauhanen M, Korpelainen JT, Hiltunen P, Brusin E, Mononen H, Maatta R, Nieminen P, Sotaniemi KA, Myllyla VV (1999) Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke 30:1875–1880
Khatri N, Man HY (2013) Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol 4:199
Krishnan E (2010) Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 49:1229–1238
Li W, Ling S, Yang Y, Hu Z, Davies H, Fang M (2014) Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett 35:104–109
Maes M, Mihaylova I, Kubera M, Ringel K (2012) Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 36:169–175
Milhavet O, McMahon HE, Rachidi W, Nishida N, Katamine S, Mange A, Arlotto M, Casanova D, Riondel J, Favier A, Lehmann S (2000) Prion infection impairs the cellular response to oxidative stress. Proc Natl Acad Sci U S A 97:13937–13942
Morris PL, Robinson RG, Raphael B (1993) Emotional lability after stroke. Aust N Z J Psychiatry 27:601–605
Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V (2014) Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014:472459
Paradiso S, Ohkubo T, Robinson RG (1997) Vegetative and psychological symptoms associated with depressed mood over the first two years after stroke. Int J Psychiatry Med 27:137–157
Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM (2013) Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 31:143–152
Rezzi S, Ramadan Z, Fay LB, Kochhar S (2007) Nutritional metabonomics: applications and perspectives. J Proteome Res 6:513–525
Rho HS, Ghimeray AK, Yoo DS, Ahn SM, Kwon SS, Lee KH, Cho DH, Cho JY (2011) Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 16:3338–3344
Sasaki M, Ohara-Nemoto Y, Tajika S, Kobayashi M, Yamaura C, Kimura S (2001) Antigenic characterisation of a novel streptococcus anginosus antigen that induces nitric oxide synthesis by murine peritoneal exudate cells. J Med Microbiol 50:952–958
Schmidtke A, Fleckenstein P, Moises W, Beckmann H (1988) Studies of the reliability and validity of the German version of the montgomery-asberg depression rating scale (MADRS). Schweiz Arch Neurol Psychiatr 139:51–65
Schubert DS, Taylor C, Lee S, Mentari A, Tamaklo W (1992) Physical consequences of depression in the stroke patient. Gen Hosp Psychiatry 14:69–76
Schwab JJ, Bialow MR, Clemmons RS, Holzer CE (1967) Hamilton rating scale for depression with medical in-patients. Br J Psychiatry 113:83–88
Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the barthel index for stroke rehabilitation. J Clin Epidemiol 42:703–709
Sinyor D, Amato P, Kaloupek DG, Becker R, Goldenberg M, Coopersmith H (1986) Post-stroke depression: relationships to functional impairment, coping strategies, and rehabilitation outcome. Stroke 17:1102–1107
Starkstein SE, Robinson RG (1989) Affective disorders and cerebral vascular disease. Br J Psychiatry 154:170–182
Sun B, Wu S, Li L, Li H, Zhang Q, Chen H, Li F, Dong F, Yan X (2009) A metabolomic analysis of the toxicity of aconitum sp. alkaloids in rats using gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 23:1221–1228
Tobe EH (2013) Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat 9:567–573
Tombaugh TN, McIntyre NJ (1992) The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40:922–935
Toso V, Gandolfo C, Paolucci S, Provinciali L, Torta R, Grassivaro N (2004) Post-stroke depression: research methodology of a large multicentre observational study (DESTRO). Neurol Sci 25:138–144
van der Greef J, Stroobant P, van der Heijden R (2004) The role of analytical sciences in medical systems biology. Curr Opin Chem Biol 8:559–565
van Ommen B, Stierum R (2002) Nutrigenomics: exploiting systems biology in the nutrition and health arena. Curr Opin Biotechnol 13:517–521
Williams LS, Jones WJ, Shen J, Robinson RL, Kroenke K (2004) Outcomes of newly referred neurology outpatients with depression and pain. Neurology 63:674–677
Wu S, Zhou F, Zhang Z, Xing D (2011) Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. Febs j 278:941–954
Xie G, Schepetkin IA, Quinn MT (2007) Immunomodulatory activity of acidic polysaccharides isolated from tanacetum vulgare L. Int Immunopharmacol 7:1639–1650
Zhang F, Jia Z, Gao P, Kong H, Li X, Lu X, Wu Y, Xu G (2010) Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol BioSyst 6:852–861
Zheng P, Gao HC, Li Q, Shao WH, Zhang ML, Cheng K, de Yang Y, Fan SH, Chen L, Fang L, Xie P (2012) Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J Proteome Res 11:1741–1748
Acknowledgments
The authors would like to thank all participants who took part in this study, and the experts at the National Center of Biomedical Analysis for providing technical assistant. This work was supported by the Chinese National Key Project of Basic Research (2009CB918301 and 2009CB918303) and the Chinese National Natural Science Foundation (81370051 and 81471155).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Xinghua Ding, Ruoxu Liu and Wenkai Li contributed equally to this work.
Electronic supplementary material
Figure S1
The GC/TOF-MS TICs of healthy subjects, PSD patients, and PSND patients. The GC/TOF-MS total ion current chromatograms (TICs) of healthy subjects' group (a), PSD (b), and PSND (c). Each peaks of TICs represented the endogenous metabolites in plasma. PSD indicates Post-stroke depression patients group, PSND indicates Post-stroke non-depression patients group. (GIF 27.7 kb)
Table S1
RPA of metabolites detected by GC/MS in human plasma A. A, The normalized intensities of metabolites in healthy control subjects, Post-stroke patients including Post-stroke depression patients (PSD) and Post-stroke non-depression patients (PSND) are expressed with their Relative peak areas (RPA). Values are presented as mean ± SD. VIPB shows variable importance in the projection obtained from the PLS-DA model with a cutoff of 1.0. a, shows VIP value and P value (Student’s t-test or Mann-whitney test) between the healthy control subjects and Post-stroke patients; b, shows VIP value and P value between the PSD patients and PSND patients. (DOCX 28.8 kb)
Table S2
RPA of sentinel metabolites among the healthy subjects' group, post-stroke patients' group and its subgroup (PSD patients and PSND patients) from test set A. A, The normalized intensities of metabolites in healthy subjects, Post-stroke patients including Post-stroke depression patients (PSD) and Post-stroke non-depression patients (PSND) are expressed with their Relative peak areas (RPA). Values are presented as mean ± SD. a, shows P value obtained from the univariate analysis between the healthy control subjects and Post-stroke patients; b, shows P value between the PSD patients and PSND patients. (DOCX 16.4 kb)
Rights and permissions
About this article
Cite this article
Ding, X., Liu, R., Li, W. et al. A metabonomic investigation on the biochemical perturbation in post-stroke patients with depressive disorder (PSD). Metab Brain Dis 31, 279–287 (2016). https://doi.org/10.1007/s11011-015-9748-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9748-z