Abstract
“Zoonoses” describe diseases that may be acquired by humans from animals. Due to the constant contact between humans and other animals, many infectious diseases are disseminated. This may happen via direct contact, such as bites or scratches, or by indirect contact, such as when eating bush meat or using contaminated animal parts. Monkeypox disease is one such zoonotic infection which is now emerging as a disease of global concern, and the World Health Organization has already labelled it a public health emergency. The virus is related to other orthopox viruses and may be further classified into two genetically separate clades, the West African and the Central African. The latter is far more pathogenic than the former. Utilizing virotransducer and virostealth proteins, the virus is able to control the host’s T-cell-mediated responses and impede the release of cytokines and chemokines.Monkeypox may be treated with tecovirimat, cidofovir, or brincidofovir, and prevention with the vaccination JYNNEOS is recommended. The disease’s fast global expansion warrants concern despite the fact that it is less fatal than that caused by the variola virus. Before the sickness reaches catastrophic proportions, we must draw on our prior experiences and act prudently. This article serves as an introduction to the monkeypox virus and its associated pathology, treatments, diagnostics, and preventative measures.
Similar content being viewed by others
Introduction
Until recently, monkeypox was only known to exist in Africa, but it has now been confirmed in more than ninety non-African countries, impacting over seventy-nine thousand individuals in just a few months. It is highly concerning that the virus has started to arise in different populations throughout the world, in places where it is unusual for it to do so. The United States of America is the worst-hit country with 28,999 reported cases and 11 deaths till date, that is, 16 November 2022 [1]. Next in the list is Brazil with 9637 reported cases. Rising occurrences of monkeypox have undoubtedly raised concerns, particularly at a time when the entire world is still healing from the pandemic shock of COVID-19. The World Health Organization (WHO) issued a global public health emergency of concern proclamation on 23 July 2022 to the ongoing monkeypox pandemic. Considering the aforementioned information, the purpose of this review paper is to familiarize the readers to the monkeypox illness, its epidemiology, pathology, diagnosis, and current therapeutic options.
Methodology
In order to write this review article, more than 200 research papers were obtained from the different search engines like Google, Science Direct, NCBI Library; websites of important organizations like WHO, CDC, and news reports, etc., using keywords like epidemiology of monkeypox, diagnosis of monkeypox, therapeutics of monkeypox disease, vaccines for prophylaxis of monkeypox, differences between monkeypox and other orthopox viruses, differences between monkeypox and smallpox, differences between monkeypox and chickenpox, differences between monkeypox and COVID-19, etc. After reading all the articles obtained from the above-mentioned sources, around 100 articles were short-listed and the information gathered from them is summarized in the form of the present review article. The map showing the geographical distribution of the monkeypox disease has been created using the data wrapper software (Fig. 1). Rest all figures have been prepared using the latest version of the Microsoft powerpoint software.
The monkeypox virus
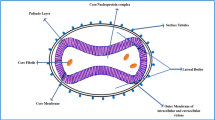
The monkeypox virus (MPXV) is a member of the Poxviridae family, specifically the Chordopoxvirinae subfamily, and it is a double-stranded DNA virus that is enveloped and is brick shaped. The virus was first discovered in primates in 1958 [2] in a laboratory in Denmark. The term “Monkeypox virus” comes from the fact that the virus was first discovered in monkeys. However, the name is misleading since monkeys are the incidental and not the natural hosts of the virus.
Different clades of the monkeypox virus
There are two clades of the virus which differ in their pathogenicity, modulation of host immune responses and rate of transmission. The monkeypox virus clade found in the Congo Basin is more fatal than the one found in West Africa. Central Africa’s case fatality rate was 10.6%, far higher than the 3.6% rate seen in the West African clade. As a whole, monkeypox is becoming more significant around the globe [3]. The Basin clade is more prone to spread and has historically caused more severe cases. The current monkeypox epidemic seems to have originated in the West Africa clade. Cameroon, which separates the two groups geographically, is the only country where both viral clades have been found [4].
Comparison of the genomes of monkeypox virus and the smallpox virus
Genetic analysis shows that the monkeypox virus is distinct from other members of the Poxviridae family, such as variola, vaccinia, ectromelia, camelpox, and cowpox. In 1958, researchers at Copenhagen’s Statens Serum Institute identified it as the agent responsible for a smallpox-like illness in cynomolgus monkeys [2]. For the first time, anything like this was uncovered. It was recognized as a novel species of orthopox virus after culture on embryonated eggs revealed pocks that resembled those of the variola virus but had other biological properties different from those of the variola virus. In 2001, a genetic comparison of MPXV and variola virus was published [5]. The essential enzymes and structural proteins are encoded by the core region of the MPXV genome, which shares 96.3% of its sequence with the variola virus. But the MPXV terminal regions that code for virulence and host range features vary greatly across strains. When comparing the MPXV genome to other orthopoxvirus genomes, it has been found that the central genomic regions are conserved and the terminal regions vary. For details, readers may refer to the work done by Shchelkunov and coworkers [5]. As with every other orthopox virus, it possesses a distinct surface epitope composition, different polypeptides, specific DNA cleavage sites, and various modifications in the double-stranded DNA genome’s long terminal repeats. The detailed genomic analysis shows that MPXV evolved from an orthopox viral progenitor apart from variola virus. The variola virus cannot easily be generated from MPXV since MPXV is not the variola virus’s direct ancestor (or vice versa). This and other data allayed worries that MPXV would transform into variola virus and reaffirmed faith in the long-term effectiveness of the smallpox immunization programme.
Similarities and differences between the monkeypox virus and the chickenpox virus
Chickenpox is caused by Herpes virus which has no correlation with the orthopox viruses but the clinical presentation of monkeypox and chickenpox appear similar which is why sometimes the disease may be misdiagnosed. However, as compared to the monkeypox, chickenpox has a brief, moderate prodrome, a mild clinical course, seldom causes lymphadenopathy, and often causes pleomorphic skin lesions to grow in a centripetal pattern, with a very low death rate [6, 7].
Epidemiology of the monkeypox disease
Between 1970 and 1979, epidemiological investigations were done in the rain forest regions of sub-Saharan Africa, where they discovered 47 cases of human monkeypox. Of these 47 occurrences, the Democratic Republic of the Congo (DRC) was responsible for 38, followed by Cameroon, the Central African Republic, Gabon, Cote d’Ivoire, Liberia, Nigeria, and Sierra Leone (together accounting for 11 incidences) [8]. Each event in the DRC had similarities that pointed to animal interaction as the likely cause; each also occurred near tropical rain forests. Of the 47 infections, only 7 were fatal. Transmission from a vulnerable person to another is the most likely explanation for illness in four of the instances. The probability of secondary transmission is 7.5% among close relatives and 3.3% among all susceptible contacts. The majority of documented instances since 1980 have come from the Democratic Republic of the Congo. For the purpose of gauging the potential of a monkeypox epidemic in central Africa, the World Health Organization (WHO) conducted surveillance in the Democratic Republic of the Congo (DRC) between 1981 and 1986 [9].
Only 13 occurrences from 1986 to 1992 were documented in the literature, and no cases from 1993 to 1995 were reported. But in Kasai-Oriental province, DRC, during 1996 and 1997, more than 500 probable cases of monkeypox were recorded [10, 11]. In spite of the fact that only a small percentage of these cases were confirmed by laboratories, the high prevalence of secondary cases (78 per cent) and the low mortality rate (one per cent to five per cent) suggest that the vast majority were in fact instances of varicella. Until 2001, when 31 individuals with monkeypox in 7 distinct illness clusters were documented in the DRC’s Equateur region, there had been no fresh reports of suspected monkeypox cases.
In total, 10 African nations and 4 other nations had then subsequently reported cases of human monkeypox. Examples include Nigeria, where the illness returned after a 40-year absence in the past decade, and the United States, where an epidemic happened in 2003. The number of cases grew at least ten times, and from young children (4 years old) in the 1970s to young adults (21 years old) in 2010–2019 [12].
The 2022 monkeypox outbreak
The current outbreak is an illustration of how quickly the virus may be transmitted by intimate contact with infected wounds. Since the beginning of May 2022, cases of the sickness have also been reported in a number of countries in which it was not endemic. Till date (16 Nov 2022) more than 79,655 cases have been recorded worldwide and less than two per cent cases are from Africa, rest all are from the non-African countries where the disease is not prevalent otherwise [1]. This is a cause of concern. By now, the disease has spread in six continents leaving just Antarctica where no case has been reported so far. These include 12 African and 93 non-African countries. The majority of reported cases had no connection to travel to an endemic nation [12]. The worldwide distribution of monkeypox cases is summarized in Table 1 and Fig. 1.
Transmission of the monkeypox virus
Squirrels (particularly Funisciurus anerythrus) living in agricultural regions have been identified in several epidemiological studies from the Democratic Republic of the Congo as the main carriers of viral transmission among local populations [13]. Funisciurus spp. squirrels showed a higher rate of MPXV seropositivity in an earlier research compared to other animals tested [14]. Gambian rats and many other non-primate and primate mammals have also been found to be seropositive [15, 16]. In conclusion, it has been determined that several animal species are vulnerable to the monkeypox virus and may serve as reservoir for the same.
Zaire was the first place where the monkeypox virus, a zoonotic orthopox DNA virus, was found in humans, back in 1970. After being discovered as a zoonotic illness in the 1970s, the MPXV virus began a pattern of frequent transmission from its reservoir hosts to humans. The transmission rate among humans was low. Most cases of this zoonosis have been documented in the Democratic Republic of the Congo among very young rural boys who participate in small-game hunting [17]. The virus may be transmitted from animals to humans by eating of bush meat, coming in contact with the body parts or body fluids of infected animals, respiratory droplets, etc.
However, the 2017 Nigerian epidemic was concentrated amongst adult males between the ages of 25 and 40 who were residents of urban or periurban areas and had no discernible contact with animal reservoirs. This might be because of sexual transmission among males. Numerous studies indicate that keeping infected people isolated reduces the spread of disease. A person can contract monkeypox virus through a variety of sexual or non-sexual contact-related methods. The latter include coming into direct contact with wounds of an infected person; coming into contact with objects and surfaces that have been contaminated by an infected person; and coming into contact with respiratory droplets or oral fluids from an infected person. Monkeypox virus may be able to penetrate the placenta, although the consequences of infection during pregnancy are yet unclear. The transmission of virus among men is corroborated by scientific testing that revealed MPXV qPCR positivity in MSM seminal fluid from Germany and other nations [18]. The different transmission routes by which the monkeypox virus can spread from animals to humans or humans to humans are summarized in Fig. 2.
Pathology and clinical features
Like other poxviruses, monkeypox has a pathogenesis that is distinguished by pronounced intracytoplasmic eosinophilic inclusions in epithelial cells [19]. The epidermis may also exhibit hyperplasia, keratinocyte necrosis, and ballooning degeneration. Ulceration and neutrophils, eosinophils, and multinucleated giant cells follow the lymphocytic inflammation seen in the dermis. As well, vascular inflammation (vasculitis) is evident.
Monkeypox in humans starts with a brief (2–3 day) febrile prodrome and proceeds to a broad rash with monomorphic, well-defined, deep-seated, and often umbilicated pustules [20]. Most skin lesions tend to show up on the face and the palms of hands and bottoms of feet. During the pustular phase, the visual features of monkeypox rash lesions clearly suggest an infection caused by the orthopox virus.
The incidence and severity of certain clinical characteristics are independent of sex, age, or vaccination status. Pre-eruptive and eruptive are the two stages of the illness’s clinical course, accordingly [21].
Pre-eruptive stage. There aren’t many chances to study patients in the pre-eruptive stage since they often seek guidance or medical assistance after several days of sickness, when lymph nodes have swelled and skin eruptions have begun. It is feasible to pinpoint the onset of the fever and rash by inquiring from the patients. It has been observed that the patient temperature ranges from 38.5 to 40.5 ℃ and reaches its peak during the second day of the illness. The patients generally experience extreme weakness, fatigue and back ache. Fever is the second most common symptom, with a headache (sometimes frontal, but often broad) being the most common symptom overall. Several people have lymph node enlargement before they develop a rash.
Eruptive stage. Except for the face, the skin rash manifests itself first on other regions of the body, such as the forearms. Lesions went through the same stages as smallpox, starting as macules and progressing through papules, vesicles, pustules, and finally umbilication, drying, and desquamation. In specific circumstances, there might have been a few or several thousand skin lesions. Vaccinated individuals had considerably fewer lesions than unvaccinated patients. In most cases, higher fever, more severe symptoms, and longer illness corresponded to higher lesion densities.
Lymph node enlargement is the only clinical indicator that may differentiate human monkeypox from smallpox and chickenpox.
The way monkeypox is currently presenting itself is also novel. Most instances of the present worldwide pandemic, including those in Nigeria, manifest as an ulcerating genital rash rather than the more widespread lesions that were seen in previous epidemics [22]. The generalized pustular rash, which is frequently mild, comes before the genital rash in clinical presentations that occur outside of Africa [23, 24]. According to this depiction, the genital area is an initial infection site that causes a discrete rash before occasionally progressing to a subsequent, disseminated illness.
Monkeypox is usually a short-lived infection, with symptoms often disappearing within two to four weeks. Severe cases are associated with the extent of virus exposure, the patient’s state, and the kind of difficulties and are more common in children. Having immunological deficiencies may make matters worse. Although smallpox immunization proved protective in the past, but people who were vaccinated more than 40 years ago may now be more vulnerable to monkeypox. Monkeypox complications can include secondary infections, bronchopneumonia, sepsis, encephalitis, and corneal infections with subsequent vision loss [25].
In the general population, the case fatality ratio of monkeypox has traditionally varied from 0 to 11%; it has been greater in young children [4].
Diagnosis
Polymerase chain reaction, often known as PCR, is now generally accepted as the gold standard for laboratory testing due to its reliability and sensitivity. Monkeypox is no exception to this rule and is best diagnosed by real-time PCR conducted on fluid isolated come from skin lesions. This fluid can also be used for viral culture, another reliable diagnostic test but this consumes longer time as compared to RT-PCR. Biopsy samples from lesions can also be used for viral detection by electron microscopy or immunohistochemistry. The serum IgG and IGM levels can also be used for detecting present or past infection [26]. However, the serological investigations do not offer any promise of monkeypox-specific infection because orthopox viruses are cross-reactive. Also, people immunized with smallpox vaccines may give false positive results [4].
Therapeutics
In the absence of any treatment option available for the monkeypox disease, FDA approved. Oral tecovirimat is currently being used as an emergency drug for the severely infected patients. Tecovirimat (TPOXX) is a drug approved by FDA for the treatment of smallpox but since the symptoms of both the diseases overlap, the drug is being used for treating MPXV-infected patients also. However, the FDA approval for the same is still pending and the clinical trials for testing the drug efficacy are going on. It is noteworthy that tecovirimat is recommended for severe cases only. As per CDC, if the drug is given to milder cases, the monkeypox virus is likely to develop resistance against it [27]. Brincidofovir and cidofovir are other drugs used for monkeypox treatment. Both the drugs stop the viral replication by inhibiting DNA polymerase enzyme. Tecovirimat on the other hand interferes with the viral envelope formation [28]. The former is lesser nephrotoxic as compared to the latter [29]. Another antiviral drug NIOCH-14 which is known to stop viral replication by serving as nucleoside is in clinical trials and approval is anticipated soon [30]. In addition to the treatment, the patients need to be fed and hydrated to keep their nutritional health in good shape [25]. Figure 3 summarizes the mode of action of different drugs used in the treatment of the monkeypox disease.
Treatment options for the monkeypox disease, and their underlying mechanisms of action. Brincidofovir and cidofovir stop the viral replication by inhibiting DNA polymerase enzyme. Tecovirimat on the other hand interferes with the viral envelope formation. Another antiviral drug NIOCH-14 is known to stop viral replication by serving as nucleoside
Immunomodulation by MPXV
Human cells obtained from monkeypox patients are resistant to T-cell receptor-mediated T-cell activation, meaning they cannot produce inflammatory cytokines. These findings point to the possibility that monkeypox generates a modulator that dampens T-cell responses in the host. It is now known that MPXV manipulates host immune responses via virotransducer and virostealth proteins. The former changes the cell’s ability to respond to infection, while the latter decreases the virus’s immunological recognition molecules, allowing it to elude the immune system. Moreover, unlike West African strains, Central African monkeypox strains preferentially downregulate host responses, including apoptosis in the host [31]. Extracellular proteins, known as viromimic proteins, function as viroreceptors to imitate cytokine and chemokine receptors, preventing glycoprotein receptors from binding, and as virokines to imitate cytokines and chemokines, disrupting host immune activation pathways and inhibiting viral reproduction [32]. Weaver and Issacs (2008) enumerated many genes encoding distinct proteins of monkeypox virus important for regulation of host immune responses, such as MOPICE in the Congo clade of monkeypox virus, which suppresses the host complement enzymes. The readers might consult the works of Weaver and Issacs (2008) for information on the remaining genes of orthopox viruses responsible for modulating the host immune responses [33].
Vaccination
The smallpox vaccination has been found in several studies to be around 85% effective at preventing monkeypox [4]. Dryvax, the smallpox vaccine of the first generation, has been rendered unusable because of the adverse side effects it may cause [34]. Similar is the case with ACAM2000, the second-generation vaccine. Recently, a third-generation vaccine developed from the Ankara strain of the modified attenuated vaccinia virus was approved for use in the prevention of monkeypox. The vaccine is marketed under the name JYNNEOOS [35, 36]. Moreover, clinical studies for the VAC6 vaccine, a vaccination of the fourth generation, are now underway [37]. All these vaccines are made from vaccinia virus since it provides cross-protection to the orthopox viruses. However, the vaccination is recommended only for immunocompromised people or for the frontline health workers who are at risk of high exposure. Because vaccinations are in short supply, it is not advised for healthy people to be vaccinated.
Future perspective
These international outbreaks of monkeypox will probably grow more frequent over time since population immunity won’t likely increase significantly in the foreseeable future. For monkeypox, a vaccination (JYNNEOS) is now available, but there is a need to look for more promising vaccine candidates. Similarly, although the treatment options are available but drug resistance might be a problem in the near future. Therefore, novel therapeutic strategies are required such as nanoparticle-based targeted drug delivery, etc. Also, the viral zoonoses are becoming a serious health issue and there is an urgent need to change our behaviour towards animals. Wild animals should remain undisturbed in their habitat, and the health of domesticated animals should be regularly monitored for any kind of pathogenesis. Also, the animals should be bred in clean and hygienic conditions to eliminate the pests like rats which can act as reservoir hosts.
Conclusion
The international outbreak has again highlighted global health inequities. In contrast to COVID-19, monkeypox did not spring upon suddenly. An unusual virus that travelled from forest species in the Democratic Republic of the Congo to villagers who hunted the animals was first discovered decades earlier.
Monkeypox is closely related to smallpox. They both belong to the same family of viruses. A smallpox infection or smallpox vaccination provides excellent protection against both smallpox and monkeypox. In other words, protection against smallpox also cross-protects against monkeypox. Since, the major population was either vaccinated for smallpox or survived an infection back in 1970s, they had some sort of immunity towards monkeypox too. However, the globe ceased immunizing individuals against smallpox in the late 1970s. Thankfully, the spread of the disease ceased among people. Therefore, immunity to smallpox and monkeypox has drastically decreased during the past forty years.
When infection does affect humans, it can be clinically difficult to identify from the chickenpox and smallpox. Severe lymphadenopathy is the most useful diagnostic clinical sign of human monkeypox; nonetheless, laboratory analysis is required for a firm diagnosis. We saw that the monkeypox virus has two clades: West African and Congo Basin. The latter causes a more serious condition. Though it was first observed in monkeys and since rodents, not monkeys, appear to be the disease’s primary natural reservoir in terms of both absolute numbers and percentages, the name “monkeypox” is rather imprecise.
The current 2022 outbreak has shown us that the stakes are really high now. A weak link anywhere is a threat everywhere. Since population immunity won’t likely increase significantly in the foreseeable future, immunising people who have come into contact with sick individuals by vaccinating them as well as accelerating our trials on currently underway therapeutics and not forgetting about making diagnostics broadly accessible must be our main focus right at the moment.
Data availability
Enquiries about data availability should be directed to the authors.
References
CDC 2022 Monkeypox in the U.S. In: Centers for Disease Control and Prevention. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html Accessed 16 Nov 2022
Magnus PV, Anderson EK, Petersen KB, Birch-Anderson A (1959) A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand 46:156–176. https://doi.org/10.1111/j.1699-0463.1959.tb00328.x
Bunge EM, Hoet B, Chen L et al (2022) The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis 16:e0010141. https://doi.org/10.1371/journal.pntd.0010141
WHO 2022 https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed 20 Nov 2022
Shchelkunov SN, Totmenin AV, Babkin IV et al (2001) Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett 509:66–70. https://doi.org/10.1016/S0014-5793(01)03144-1
Jezek Z, Szczeniowski MV, Paluku KM, Mutombo M, Grab B (1988) Human monkeypox: confusion with chickenpox. Acta Trop 45:297–307
Breman JG (2000) Monkeypox: an emerging infection for humans? In: Craig WA, Hughes JM (eds) ScheldMW, vol Emerging infections 4. ASM Press, Washington, pp 45–76
Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I (1980) Human monkeypox, 1970–79. Bull World Health Organ 58:165–182
Jezek Z, Grab B, Paluku KM, Szczeniowski MV (1988) Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop Geogr Med 40:73–83
Heymann DL, Szczeniowski M, Esteves K (1988) Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull 54:693–702. https://doi.org/10.1093/oxfordjournals.bmb.a011720
Hutin YJ, Williams RJ, Malfait P et al (2001) Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis 7:434–438. https://doi.org/10.3201/eid0703.010311
WHO 2022 Multi-country monkeypox outbreak: situation update. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393. Accessed 20 Oct 2022
Khodakevich L, Jezek Z, Kinzanzka K (1986) Isolation of monkeypox virus from wild squirrel infected in nature. The Lancet 327:98–99. https://doi.org/10.1016/S0140-6736(86)90748-8
Khodakevich L, Jezek Z, Messinger D (1988) Monkeypox virus: ecology and public health significance. Bull World Health Organ 66:747–752
Larkin M (2003) Monkeypox spreads as US public-health system plays catch-up. Lancet Infect Dis 3:461. https://doi.org/10.1016/s1473-3099(03)00713-8
CDC 2022 CDC Confirms Monkeypox in Rodents. https://www.cdc.gov/media/pressrel/r030702.htm. Accessed 20 Oct 2022
Nolen LD, Osadebe L, Katomba J et al (2015) Introduction of monkeypox into a community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg 93:410–415. https://doi.org/10.4269/ajtmh.15-0168
Ogoina D, Iroezindu M, James HI et al (2022) Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis 71:e210–e214. https://doi.org/10.1093/cid/ciaa143
Zaucha GM, Jahrling PB, Geisbert TW et al (2001) The Pathology of Experimental Aerosolized Monkeypox Virus Infection in Cynomolgus Monkeys (Macaca fascicularis). Lab Invest 81:1581–1600. https://doi.org/10.1038/labinvest.3780373
Centers for Disease Control and Prevention (CDCP) 2022 https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html. Accessed on 15 October 2022
Jezek Z, Szczeniowski M, Paluku KM, Mutombo M (1987) Human monkeypox: clinical features of 282 patients. J Infect Dis 156:293–298. https://doi.org/10.1093/infdis/156.2.293
Antinori A, Mazzotta V, Vita S et al (2022) Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill 27:2200421. https://doi.org/10.2807/1560-7917.ES.2022.27.22.2200421
Hammerschlag Y, MacLeod G, Papadakis G et al (2022) Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill 27:2200411. https://doi.org/10.2807/1560-7917.ES.2022.27.22.2200411
Iñigo Martínez J, Gil Montalbán E, Jiménez Bueno S et al (2022) Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance. https://doi.org/10.2807/1560-7917.ES.2022.27.27.2200471
Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW (2017) Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 9:380. https://doi.org/10.3390/v9120380
Cheema AY, Ogedegbe OJ, Munir M et al (2022) Monkeypox: a review of clinical features, diagnosis, and treatment. Cureus. https://doi.org/10.7759/cureus.26756
Beatty NL, Small C, Degener T, Henao Martinez AF (2022) Monkeypox infection and resolution after treatment with tecovirimat in two patients with HIV disease. Therapeutic Advances in Infection 9:204993612211383. https://doi.org/10.1177/20499361221138349
Rizk JG, Lippi G, Henry BM et al (2022) Prevention and treatment of monkeypox. Drugs 82:957–963. https://doi.org/10.1007/s40265-022-01742-y
Gong Q, Wang C, Chuai X, Chiu S (2022) Monkeypox virus: a re-emergent threat to humans. Virologica Sinica 37:477–482. https://doi.org/10.1016/j.virs.2022.07.006
World Health Organization (2022) WHO advisory committee on variola virus research: report of the twenty-third meeting, virtual meeting, 3–4 November 2021. World Health Organization, Geneva
Kindrachuk J, Arsenault R, Kusalik A et al (2012) Systems Kinomics Demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol Cell Proteomics 11:M111.015701. https://doi.org/10.1074/mcp.M111.015701
Kaler J, Hussain A, Flores G et al (2022) Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. https://doi.org/10.7759/cureus.26531
Weaver JR, Isaacs SN (2008) Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev 225:96–113. https://doi.org/10.1111/j.1600-065X.2008.00691.x
Belongia EA, Naleway AL (2003) Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin Med Res 1:87–92. https://doi.org/10.3121/cmr.1.2.87
Medcalf S, Bilek L, Hartman T et al (2015) Smallpox vaccination of laboratory workers at US Variola testing sites. Emerg Infect Dis 21:1437–1439. https://doi.org/10.3201/eid2108.140956
Isaacs SN, Friedman HM 2022 Vaccines to prevent smallpox, monkeypox, and other orthopoxviruses https://www.uptodate.com/contents/vaccines-to-prevent-smallpox-monkeypox-and-other-orthopoxviruses?sectionName=Complications&topicRef=8295&anchor=H14&source=see_link#:~:text=MODIFIED%20VACCINIA%20ANKARA%20(NON%2DREPLICATING)%20VACCINE%20%E2%80%94%20The%20modified,of%20both%20smallpox%20and%20monkeypox. Accessed 15 Oct 2022
Abdelaal A, Reda A, Lashin BI et al (2022) Preventing the next pandemic: is live vaccine efficacious against monkeypox, or is there a need for killed virus and mRNA vaccines? Vaccines 10:1419. https://doi.org/10.3390/vaccines10091419
Oliviero, H 2022 Health officials announce first monkeypox case in woman in Georgia. The Atlanta Journal-Constitution. https://www.ajc.com/news/atlanta-news/breaking-news-health-officials-announce-first-monkeypox-case-in-woman-in-georgia/HWJ3BIHZIZBY7JXROH3RKLAM3Y/#:~:text=A%20woman%20in%20Georgia%20has,with%20monkeypox%2C%20according%20to%20DPH. Accessed on 15 Oct 2022
WHO 2022 India confirms first case of monkeypox in WHO South-East Asia Region. https://www.who.int/southeastasia/news/detail/15-07-2022-india-confirms-first-case-of-monkeypox-in-who-south-east-asia-region. Accessed 20 Oct 2022
VOA 2022 India Reports First Death Due to Monkeypox. In: VOA. https://www.voanews.com/a/india-reports-first-death-due-to-monkeypox-/6683370.html. Accessed 20 Oct 2022
Reuters 2022 Indonesia confirms first monkeypox case in citizen returning from abroad. Reuters https://www.reuters.com/world/asia-pacific/indonesia-brief-media-first-confirmed-monkeypox-case-2022-08-20/. Accessed on 15 Oct 2022
Huaxia 2022 Iran reports 1st monkeypox case. https://english.news.cn/20220817/0e997ee3368e4eeda9558ca39eac03ec/c.html#:~:text=TEHRAN%2C%20Aug.,skin%20lesions%2C%20according%20to%20IRNA. Accessed on 15 Oct 2022
The Hindu 2022 Israel reports first case of monkeypox, suspects other https://www.thehindu.com/news/international/israel-reports-first-case-of-monkeypox-suspects-others/article65446444.ece#comments_65446444. Accessed on 15 Oct 2022
Reuters 2022 UAE announces first case of monkeypox in the country. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/uae-announces-first-case-monkeypox-country-wam-2022-05-24/#:~:text=May%2024%20(Reuters)%20%2D%20The,Gulf%20country%20from%20West%20Africa. Accessed on 15 Oct 2022
The straits times 2022 Thailand's first monkeypox case found in Phuket. https://www.straitstimes.com/asia/se-asia/thailands-first-monkeypox-case-found-in-phuket. Accessed on 15 Oct 2022
Reuters 2022 Philippines reports first monkeypox case, traces 10 close contac.t https://www.reuters.com/world/asia-pacific/philippines-detects-first-case-monkeypox-2022-07-29/. Accessed on 15 Oct 2022
Reuters 2022 Japan detects first monkeypox case. https://www.reuters.com/world/asia-pacific/first-case-monkeypox-japan-detected-tokyo-ntv-citing-govt-source-2022-07-25/#:~:text=TOKYO%2C%20July%2025%20(Reuters),%2C%20fever%2C%20headache%20and%20fatigue. Accessed on 15 Oct 2022
Fatima S 2022 Third in Gulf, Qatar reports first case of monkeypox. In: The Siasat Daily. https://www.siasat.com/third-in-gulf-qatar-reports-first-case-of-monkeypox-2375170/. Accessed 15 Oct 2022
Salari F 2022 First case of monkeypox detected in Qatar. In: Doha News | Qatar. https://dohanews.co/first-case-of-monkeypox-detected-in-qatar/. Accessed 15 Oct 2022
Huaxia 2022 Saudi Arabia detects first monkeypox case. https://english.news.cn/20220715/ba70943e90e9446488f7ad029138dde0/c.html#:~:text=RIYADH%2C%20July%2014%20(Xinhua),said%20the%20Saudi%20Health%20Ministry. Accessed 15 Oct 2022
Jang YR, Lee M, Shin H et al (2022) The first case of monkeypox in the Republic of Korea. J Korean Med Sci 37:e224. https://doi.org/10.3346/jkms.2022.37.e224
Reuters 2022 Singapore confirms first local case of monkeypox infection https://www.reuters.com/world/asia-pacific/singapore-confirms-first-local-case-monkeypox-infection-2022-07-06/. Accessed 15 Oct 2022
Reuters 2022 Lebanon announces first monkeypox case - state news agency https://www.reuters.com/world/middle-east/lebanon-announces-first-monkeypox-case-state-news-agency-2022-06-20/. Accessed 15 Oct 2022
India Today 2022 China reports its first monkeypox case in person who arrived from abroad. In: India Today. https://www.indiatoday.in/world/story/monkeypox-virus-mainland-china-chongqing-city-first-case-infection-low-transmission-risk-2001279-2022-09-17. Accessed 20 Oct 2022
Healthalert 2022 The official website for the latest health developments, Kingdom of Bahrain. https://healthalert.gov.bh/en/article/Coronavirus, COVID-19, Bahrain Coronavirus, TeamBahrain. Accessed 20 Oct 2022
Jordan 2022 Jordan records first monkeypox case. In: Jordan Times. https://jordantimes.com/news/local/jordan-records-first-monkeypox-case. Accessed 15 Oct 2022
Yang Z-S, Lin C-Y, Urbina AN et al (2022) The first case of monkeypox virus infection detected in Taiwan: awareness and preparation. Int J Infect Dis 122:991–995. https://doi.org/10.1016/j.ijid.2022.07.051
Reuters 2022 Turkey Records First Case of Monkeypox - Health Minister https://www.reuters.com/world/middle-east/turkey-records-first-case-monkeypox-health-minister-2022-06-30/. Accessed 15 Oct 2022
Vaughan AM, Cenciarelli O, Colombe S et al (2022) A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 march to 23 august 2022. Euro Surveill 27:2200620. https://doi.org/10.2807/1560-7917.ES.2022.27.36.2200620
Reuters 2022 Germany detects first case of monkeypox, military medical service says https://www.reuters.com/business/healthcare-pharmaceuticals/germany-detects-first-case-monkeypox-military-medical-service-2022-05-20/. Accessed 15 Oct 2022
Rogulj D 2022 First Case of Monkeypox in Croatia Confirmed https://www.total-croatia-news.com/news/63834-monkeypox-in-croatia. Accessed 15 Oct 2022
News desk 2022 Lithuania reports 1st monkeypox case http://outbreaknewstoday.com/lithuania-reports-1st-monkeypox-case-54849/#:~:text=The%20Ministry%20of%20Health%20reported,aged%2040%2D49%20age%20group. Accessed 15 Oct 2022
JCA 2022 First Monkeypox Case Confirmed in Luxembourg https://chronicle.lu/category/medical/41404-first-monkeypox-case-confirmed-in-luxembourg. Accessed 15 Oct 2022
Times of Malta 2022 Malta detects first monkeypox case https://timesofmalta.com/articles/view/malta-detects-first-monkeypox-case.958072. Accessed 15 Oct 2022
Reuters 2022 Cyprus records its first monkeypox case - health ministry https://www.reuters.com/world/europe/cyprus-records-its-first-monkeypox-case-health-ministry-2022-08-02/. Accessed 15 Oct 2022
BBC 2022 First case of monkeypox in Republic of Ireland https://www.bbc.com/news/articles/cqlvpk00k42o. Accessed 15 Oct 2022
Interfax 2022 First monkeypox case identified in Moldova https://interfax.com/newsroom/top-stories/82057/#:~:text=CHISINAU.,interest%20of%20work%2C%20Nemerenko%20said. Accessed 15 Oct 2022
HelloMonaco 2022 First Case of Monkey Pox Confirmed in Monaco. In: HelloMonaco. https://www.hellomonaco.com/news/latest-news/first-case-of-monkey-pox-detected-in-monaco-what-you-need-to-know/. Accessed 15 Oct 2022
ERR News 2022 Latvia gets first confirmed monkeypox case https://news.err.ee/1608619159/latvia-gets-first-confirmed-monkeypox-case. Accessed 15 Oct 2022
Reuters 2022 Italy reports first case of monkeypox infection, two more suspected https://www.reuters.com/business/healthcare-pharmaceuticals/italy-reports-first-case-monkeypox-infection-two-more-suspected-2022-05-19/. Accessed 15 Oct 2022
Reuters 2022 Czech Republic detects its first case of monkeypox https://www.reuters.com/world/europe/czech-republic-detects-its-first-case-monkeypox-2022-05-24/. Accessed 15 Oct 2022
UNIINDIA 2022 Montenegro confirms first monkeypox case. https://www.uniindia.com/montenegro-confirms-first-monkeypox-case/world/news/2791807.html. Accessed 15 Oct 2022
CNA 2022 First case of monkeypox confirmed in Finland https://www.channelnewsasia.com/world/first-case-monkeypox-confirmed-finland-2711436#:~:text=HELSINKI%3A%20Finland%20has%20confirmed%20its,to%20confirm%20it%20was%20monkeypox. Accessed 15 Oct 2022
ANI 2022 France reports first case of monkeypox https://theprint.in/world/france-reports-first-case-of-monkeypox/964452/. Accessed 15 Oct 2022
Reuters 2022 Greece detects first case of monkeypox infection https://www.reuters.com/world/europe/greece-detects-first-case-monkeypox-infection-2022-06-08/. Accessed 15 Oct 2022
Reuters 2022 Norway reports its first case of monkeypox https://www.reuters.com/world/europe/norway-reports-its-first-case-monkeypox-2022-05-31/. Accessed 15 Oct 2022
Ministerie van Volksgezondheid W en S (MVW) 2022 Monkeypox cases must be reported for effective disease control - News item - Government.nl. https://www.government.nl/latest/news/2022/05/24/monkeypox-cases-must-be-reported. Accessed on 15 Oct 2022
Reuters 2022 Hungary reports first case of monkeypox https://www.reuters.com/world/europe/hungary-reports-first-case-monkeypox-2022-05-31/. Accessed on 15 Oct 2022
Grapevine 2022 From Iceland — First Monkeypox Cases Diagnosed In Iceland. In: The Reykjavik Grapevine. https://grapevine.is/news/2022/06/09/first-monkeypox-cases-diagnosed-in-iceland/. Accessed 15 Oct 2022
Reuters 2022 Austria's first case of monkeypox confirmed, Vienna health authority says https://www.reuters.com/world/europe/austrias-first-case-monkeypox-confirmed-vienna-health-authority-says-2022-05-22/. Accessed on 15 Oct 2022
NFP 2022 Poland confirms first case of monkeypox https://notesfrompoland.com/2022/06/10/poland-confirms-first-case-of-monkeypox/. Accessed 15 Oct 2022
IANS 2022 Portugal begins vaccination for close contact of Monkeypox patients https://www.business-standard.com/article/international/portugal-begins-vaccination-for-close-contact-of-monkeypox-patients-122072200550_1.html#:~:text=The%20Portuguese%20Health%20authority%20on,week%2C%20Xinhua%20news%20agency%20reported. Accessed 15 Oct 2022
UNIINDIA 2022 Romania reports one new monkeypox case. http://www.uniindia.com/romania-reports-one-new-monkeypox-case/world/news/2786241.html. Accessed 15 Oct 2022
CNBC 2022 Russia reports first case of monkeypox https://www.cnbctv18.com/healthcare/russia-monkeypox-first-case-reported-14119102.htm. Accessed 15 Oct 2022
EPDATA 2022 Monkeypox cases in Spain (may 18) and in the world, statistics and graphs https://www.epdata.es/datos/viruela-mono-espana-mundo-datos-graficos/662. Accessed 15 Oct 2022
AFP 2022 Sweden reports first case of monkeypox https://www.thenews.com.pk/latest/959055-sweden-reports-first-case-of-monkeypox. Accessed 15 Oct 2022
FOPH 2022 Monkeypox https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/affenpocken.html#:~:text=The%20first%20reported%20case%20of,Switzerland%20on%2019%20May%202022. Accessed 15 Oct 2022
ERR News 2022 First case of monkeypox diagnosed in Estonia https://news.err.ee/1608642541/first-case-of-monkeypox-diagnosed-in-estonia. Accessed 15 Oct 2022
Reuters 2022 Belgium detects first two monkeypox cases https://www.reuters.com/business/healthcare-pharmaceuticals/belgium-detects-first-two-monkeypox-cases-2022-05-20/. Accessed 15 Oct 2022
Huaxia 2022 BiH confirms first monkeypox case -Source: Xinhua https://english.news.cn/20220714/a3455888d85f4d67a3ab20c98b07f9c4/c.html#:~:text=SARAJEVO%2C%20July%2014%20(Xinhua),tested%20positive%20for%20the%20virus. Accessed 15 Oct 2022
Reuters 2022 Bulgaria reports first monkeypox cases https://www.reuters.com/business/healthcare-pharmaceuticals/bulgaria-reports-first-monkeypox-cases-2022-06-23/. Accessed 15 Oct 2022
BETA 2022 Public Health Institute says 31 monkeypox cases in Serbia https://rs.n1info.com/english/news/public-health-institute-says-31-monkeypox-cases-in-serbia/#:~:text=The%20Serbian%20Public%20Health%20Institute,men%20aged%2021%20to%2044. Accessed 15 Oct 2022
Reuters 2022 Slovakia confirms first case monkeypox public health authority. https://www.reuters.com/world/europe/slovakia-confirms-first-case-monkeypox-public-health-authority-2022-07-07/. Accessed 15 Oct 2022
Slovenia times 2022 Slovenia confirms 35 monkeypox cases so far. https://sloveniatimes.com/slovenia-confirms-35-monkeypox-cases-so-far/#:~:text=The%20first%20case%20in%20Slovenia,been%20to%20Spain%20as%20well. Accessed 15 Oct 2022
DHA 2022 Monkey pox. https://www.sst.dk/en/English/Expertise-and-guidance/General-public/Monkeypox#:~:text=Currently%2C%20Denmark%20and%20the%20rest,was%20confirmed%20in%20May%202022. Accessed 15 Oct 2022
Gibraltar Chronicle 2022 GHA confirms first case of monkeypox in Gibraltar. https://www.chronicle.gi/gha-confirms-first-case-of-monkeypox-in-gibraltar/. Accessed 15 Oct 2022
Ukrinform 2022 First monkeypox case confirmed in Ukraine. https://www.ukrinform.net/rubric-society/3572315-first-monkeypox-case-confirmed-in-ukraine.html. Accessed 20 Oct 2022
ECDC 2022 Monkeypox cases reported in UK and Portugal. https://www.ecdc.europa.eu/en/news-events/monkeypox-cases-reported-uk-and-portugal#:~:text=The%20first%20case%20was%20reported,case%20reported%20on%207%20May. Accessed 15 Oct 2022
AfricaNews 2022 South Africa reports first case of monkeypox. In: Africanews. https://www.africanews.com/2022/06/23/south-africa-reports-first-case-of-monkeypox/. Accessed 15 Oct 2022
News desk 2022 Central African Republic reports monkeypox outbreak since March. In: Outbreak News Today. http://outbreaknewstoday.com/central-african-republic-reports-monkeypox-outbreak-since-march-28671/. Accessed 15 Oct 2022
Reuters 2022 Liberia reports first monkeypox case since 2018 https://www.reuters.com/world/africa/liberia-reports-first-monkeypox-case-since-2018-2022-07-25/. Accessed 15 Oct 2022
News desk 2022 Cameroon monkeypox: Follow-up and more details http://outbreaknewstoday.com/cameroon-monkeypox-follow-up-and-more-details-15866/. Accessed 15 Oct 2022
News desk 2022 Africa: Benin reports 3 monkeypox cases http://outbreaknewstoday.com/africa-benin-reports-3-monkeypox-cases-16828/. Accessed 15 Oct 2022
News desk 2022 Ghana reports monkeypox outbreak, 5 confirmed cases http://outbreaknewstoday.com/ghana-reports-monkeypox-outbreak-5-confirmed-cases-47190/. Accessed 15 Oct 2022
AfricaNews 2022 Morocco reports first confirmed case of monkeypox - Health Ministry- https://www.africanews.com/2022/06/03/morocco-reports-first-confirmed-case-of-monkeypox-health-ministry//. Accessed 15 Oct 2022
Prasad R 2022 Mistake of ignoring monkeypox outbreak in Nigeria. The Hindu. https://www.thehindu.com/sci-tech/science/mistake-of-ignoring-monkeypox-outbreak-in-nigeria/article65467484.ece. Accessed 15 Oct 2022
SudanTribune 2022 Sudan reports first monkeypox case. In: Sudan Tribune. https://sudantribune.com/article262136/. Accessed 15 Oct 2022
Medicalxpress 2022 Egypt records its first monkeypox case. https://medicalxpress.com/news/2022-09-egypt-monkeypox-case.html. Accessed 20 Oct 2022
Online T 2022 First monkeypox case detected in Mozambique. In: Tribune Online. https://tribuneonlineng.com/first-monkeypox-case-detected-in-mozambique/. Accessed 20 Oct 2022
News desk 2022 Canada: Yukon reports 1st monkeypox case http://outbreaknewstoday.com/canada-yukon-reports-1st-monkeypox-case-28821/#:~:text=The%20first%20monkeypox%20case%20has,Sudit%20Ranade.&text=%E2%80%9COn%20Thursday%2C%20July%2021%2C,was%20confirmed%20in%20the%20territory. Accessed 15 Oct 2022
News desk 2022 Costa Rica reports 1st monkeypox case http://outbreaknewstoday.com/costa-rica-reports-1st-monkeypox-case-61307/#:~:text=The%20Costa%20Rica%20Ministry%20of,is%20considered%20a%20imported%20case. Accessed 15 Oct 2022
Loop News 2022 Monkeypox case confirmed in Martinique https://caribbean.loopnews.com/content/monkeypox-case-confirmed-martinique. Accessed 15 Oct 2022
Fuentes M 2022 Cuba confirms first monkeypox case in visitor from Italy. Reuters https://www.reuters.com/world/americas/cuba-confirms-first-monkeypox-case-visitor-italy-2022-08-21/. Accessed 15 Oct 2022
Reuters 2022 Mexico confirms first case of monkeypox https://www.reuters.com/world/americas/mexico-confirms-first-case-monkeypox-health-official-2022-05-28/#:~:text=MEXICO%20CITY%2C%20May%2028%20(Reuters,Lopez%2DGatell%20said%20on%20Twitter. Accessed 15 Oct 2022
Ministry of Health & Wellness, Jamaica 2022 First Local Monkeypox Case confirmed in Jamaica https://www.moh.gov.jm/first-local-monkeypox-case-confirmed-in-jamaica/#:~:text=Jamaica%20has%20confirmed%20a%20third,the%20two%20previously%20announced%20cases. Accessed 15 Oct 2022
The limited times 2022 https://newsrnd.com/news/2022-08-10-monkeypox-virus-arrives-in-greenland.Hkl8mDTlR9.html. Accessed 15 Oct 2022
Moreno E 2022 Panama reports country’s first monkeypox case. Reutershttps://www.reuters.com/business/healthcare-pharmaceuticals/panama-reports-countrys-first-monkeypox-case-2022-07-05/#:~:text=PANAMA%20CITY%2C%20July%205%20(Reuters,Panama's%20health%20ministry%20said%20Tuesday. Accessed 15 Oct 2022
Dept E 2022 ARS confirms first case of monkeypox on French side. In: The Daily Herald. https://www.thedailyherald.sx/islands/ars-confirms-first-case-of-monkeypox-on-french-side. . Accessed 15 Oct 2022
Huaxia 2022 Guatemala confirms first case of monkeypox. https://english.news.cn/20220804/c20fe8d0f1a447a4b88f418f95137d10/c.html. Accessed 15 Oct 2022
UNIINDIA 2022 Honduras logs 4 monkey pox cases- https://www.uniindia.com/~/honduras-logs-4-monkeypox-cases/World/news/2811781.html. Accessed 15 Oct 2022
News desk 2022 Bermuda reports 1st monkeypox case http://outbreaknewstoday.com/bermuda-reports-1st-monkeypox-case-58966/#:~:text=The%20Bermuda%20Ministry%20of%20Health,case%20of%20monkeypox%20on%20Thursday.&text=The%20patient%20is%20in%20isolation,who%20may%20have%20been%20exposed. Accessed 15 Oct 2022
Reuters 2022 Dominican Republic confirms first case of monkeypox https://www.reuters.com/world/americas/dominican-republic-confirms-first-case-monkeypox-2022-07-07/#:~:text=SANTO%20DOMINGO%2C%20July%206%20(Reuters,Daniel%20Rivera%20said%20on%20Wednesday. Accessed 15 Oct 2022
Shong DD 2022 The Bahamas confirms first case of monkeypox. Another suspected case is under investigation. https://caribbean.loopnews.com/content/bahamas-confirms-first-case-monkeypox-613674. Accessed 15 Oct 2022
The cleaner 2022 Barbados Saturday confirmed its first case of the monkeypox virus with officials indicating that the island is fully prepared to handle the situation. https://jamaica-gleaner.com/article/caribbean/20220716/barbados-records-first-case-monkeypox. Accessed 15 Oct 2022
Press 2022 First case of monkeypox has been identified on French, Saint Martin http://www.sintmaartengov.org/PressReleases/Pages/First-case-of-Monkeypox-has-been-identified-on-French-Saint-Martin.aspx. Accessed 15 Oct 2022
Balevic K 2022 Here's how the US handled its first ever monkeypox outbreak that infected dozens of people across several states https://www.businessinsider.in/international/news/hereaposs-how-the-us-handled-its-first-ever-monkeypox-outbreak-that-infected-dozens-of-people-across-severalstates/slidelist/91728937.cms. Accessed 01 Oct 2022
Amandala 2022 1st case of monkeypox identified in El Salvador. In: Amandala Newspaper. https://amandala.com.bz/news/1st-case-of-monkeypox-identified-in-el-salvador/. Accessed 20 Oct 2022
IANS 2022 Argentina confirms first case of monkeypox in man who came from Spain https://www.business-standard.com/article/international/argentina-confirms-first-case-of-monkeypox-in-man-who-came-from-spain-122052800080_1.html#:~:text=Argentina%20has%20confirmed%20its%20first,Spain%2C%20the%20Health%20Ministry%20said&text=Photo%3A%20Shutterstock-,Argentina%20has%20confirmed%20its%20first%20case%20of%20monkeypox%20in%20a,the%20Health%20Ministry%20has%20said. Accessed 15 Oct 2022
Overheid A 2022 First positive Monkeypox case in Aruba https://www.government.aw/news/news_47033/item/first-positive-monkeypox-case-in-aruba_60314.html#:~:text=ORANJESTAD%20%E2%80%93%20Aruba%20has%20its%20first,a%20suspicious%20case%20under%20investigation. Accessed 15 Oct 2022
Reuters 2022 Chile reports first case of monkeypox- https://www.reuters.com/world/americas/chile-reports-first-case-monkeypox-2022-06-17/. Accessed 15 Oct 2022
Reuters 2022 Colombia reports first cases of monkeypox- https://www.reuters.com/business/healthcare-pharmaceuticals/colombia-reports-first-cases-monkeypox-2022-06-24/. Accessed 15 Oct 2022
Merco Press 2022 Paraguay has first case of monkeypox confirmed https://en.mercopress.com/2022/08/26/paraguay-first-case-of-monkeypox-confirmed Accessed 15 Oct 2022
Lima 2022 Peru reports first monkeypox case http://www.uniindia.com/peru-reports-first-monkeypox-case/world/news/2766711.html. Accessed 15 Oct 2022
DPI 2022 First case of monkeypox confirmed in Guyana. In: Department of Public Information, Guyana. https://dpi.gov.gy/first-case-of-monkeypox-confirmed-in-guyana/. Accessed 15 Oct 2022
Huaxia 2022 Bolivia sees 2 new monkeypox cases, steps up control measures. https://english.news.cn/20220805/666837fe5d5548dab24eec15e9682851/c.html#:~:text=The%20country's%20first%20monkeypox%20case,who%20recently%20traveled%20to%20Brazil. Accessed 15 Oct 2022
Huaxia 2022 Ecuador confirms first case of monkeypox. https://english.news.cn/20220707/4c640f39acf4474c8c7fb3bcfd71c392/c.html. Accessed 15 Oct 2022
AFP-TOI 2022 Brazil confirms its first monkeypox https://timesofindia.indiatimes.com/world/rest-of-world/brazil-confirms-its-first-monkeypox-case/articleshow/92119319.cms. Accessed 15 Oct 2022
Curaçao Chronicles 2022 Confirmed: First monkeypox infection registered on Curaçao https://www.curacaochronicle.com/post/local/confirmed-first-monkeypox-infection-registered-on-curacao/. Accessed 15 Oct 2022
Merco Press 2022 Uruguay has first case of monkeypox confirmed https://en.mercopress.com/2022/07/30/uruguay-has-first-case-of-monkeypox-confirmed. Accessed 15 Oct 2022
Reuters 2022 Venezuela confirms first case of monkeypox https://www.reuters.com/business/healthcare-pharmaceuticals/venezuela-confirms-first-case-monkeypox-2022-06-12/. Accessed 15 Oct 2022
France 2022 A case of monkey pox detected in New Caledonia https://www.france24.com/fr/info-en-continu/20220712-un-cas-de-variole-du-singe-d%C3%A9tect%C3%A9-en-nouvelle-cal%C3%A9donie. Accessed 15 Oct 2022
IANS 2022 New Zealand Registers First Monkeypox Case In Auckland https://www.india.com/news/world/new-zealand-registers-first-monkeypox-case-in-auckland-5503946/. Accessed 15 Oct 2022
Woolley S 2022 Australia’s first confirmed monkeypox case recorded in Victoria https://7news.com.au/news/public-health/warning-in-vic-for-rare-monkeypox-disease-c-6867171. Accessed on 15 Oct 2022
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MT and PD wrote the manuscript; MT prepared the figures; and Dr.TK and Dr.RCS reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakur, M., Das, P., Sobti, R.C. et al. Human monkeypox: epidemiology, transmission, pathogenesis, immunology, diagnosis and therapeutics. Mol Cell Biochem 478, 2097–2110 (2023). https://doi.org/10.1007/s11010-022-04657-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04657-0