Abstract
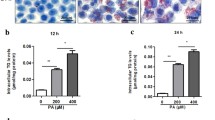
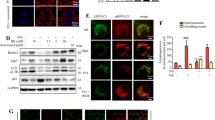
Nonalcoholic fatty liver disease (NAFLD) is related to elevated cytoplasmic calcium signaling in hepatocytes, which may be mediated by store-operated calcium channel (SOCC) and inositol triphosphate receptor (IP3R). However, the regulatory effect of calcium signaling on lipid accumulation and degeneration in hepatocytes and the underlying molecular mechanism remain unknown. Autophagy inhibition promotes lipid accumulation and steatosis in hepatocytes. However, the association between elevated calcium signaling and autophagy inhibition in hepatocytes and its effect on hepatocyte fatty lesions remain unclear. Here, we established a mouse hepatocyte fatty gradient model using oleic acid. SOCC and IP3R channel opening and cytoplasmic calcium levels gradually increased with the hepatocyte pimelosis degree, whereas autophagy gradually decreased. We also established an optimal oleic acid (OOA) hepatocyte model, observing significantly increased SOCC and IP3R channel opening and calcium influx alongside significantly decreased autophagy and aggravated cellular fatty lesion. Calcium channel blockers (CCBs) and calcium channel gene silencing reagents (CCGSRs), respectively, reversed these effects, indicating that elevated cytoplasmic calcium signaling promotes NAFLD occurrence and the development by inhibiting hepatocyte autophagy. In the OOA model, upregulated extracellular regulated protein kinases 1/2 (ERK1/2), which can be regulated by SOCC and IP3R proteins transient receptor potential canonical 1 (TRPC1)/IP3R with elevated cytoplasmic calcium signaling, over-inhibited forkhead/winged helix O (FOXO) signaling and over-activated mammalian target of rapamycin complex 1 (mTORC1) signaling. Over-inhibited FOXO signaling significantly downregulated autophagy-related gene 12, which inhibits autophagosome maturation, while over-activated mTORC1 signaling over-inactivated Unc-51 like autophagy activating kinase 1, which inhibits preautophagosome formation. CCBs and CCGSRs recovered autophagy by significantly downregulating ERK1/2 to block abnormal changes in FOXO and mTORC1 signaling. Our findings indicate that upregulated SOCC and IP3R channels and subsequent elevated cytoplasmic calcium signaling in hepatocyte fatty lesions inhibits hepatocyte autophagy through (TRPC1/IP3R)/ERK/(FOXO/mTORC1) signaling pathways, causes lipid accumulation and degeneration in hepatocytes, and promotes NAFLD occurrence and development.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2-APB:
-
2-Aminoethoxydiphenyl borate
- ATG:
-
Autophagy-related gene
- BECLIN1:
-
Yeast ATG6 homolog
- CCB:
-
Calcium channel blocker
- CCGSR:
-
Calcium channel gene silencing reagent
- DMEM:
-
Dulbecco's Modified Eagle Medium
- ECL:
-
Electrochemiluminescence
- EP tubes:
-
Eppendorf micro test tubes
- ER:
-
Endoplasmic reticulum
- ERK1/2:
-
Extracellular regulated protein kinases 1/2
- FBS:
-
Fetal bovine serum
- FOXO:
-
Forkhead/winged helix O
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GOA:
-
Gradient oleic acid
- HCC:
-
Hepatocellular carcinoma
- IP3R:
-
Inositol triphosphate receptor
- LC3B:
-
Microtubule-associated protein 1- light chain 3 B
- MAPK:
-
Mitogen-activated protein kinase
- mTORC1:
-
Mammalian target of rapamycin complex 1
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- OD:
-
Optical density
- OOA:
-
Optimal oleic acid
- ORAI1:
-
Calcium release-activated calcium channel protein 1
- p:
-
Phosphorylated
- PBS:
-
Phosphate-buffered saline
- RT:
-
Reverse transcription
- SOCC:
-
Store-operated calcium channel
- STIM1:
-
Stromal interaction molecule 1
- t:
-
Total
- TBST:
-
Tris-buffered saline Tween
- TG:
-
Triglyceride
- TRPC1:
-
Transient receptor potential canonical 1
- TSC1/2:
-
Tuberous sclerosis 1/2
- ULK1:
-
Unc-51 like autophagy activating kinase 1
- VHP:
-
Verapamil HCl
- VPS:
-
Vacuolar protein sorting
References
Huang TD, Behary J, Zekry A (2020) Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J 50(9):1038–1047. https://doi.org/10.1111/imj.14709
Ali ES, Rychkov GY, Barritt GJ (2019) Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma. Cell Calcium 82:102057. https://doi.org/10.1016/j.ceca.2019.102057
Barritt GJ, Litjens TL, Castro J, Aromataris E, Rychkov GY (2009) Store-operated Ca2+ channels and microdomains of Ca2+ in liver cells. Clin Exp Pharmacol Physiol 36:77–83. https://doi.org/10.1111/j.1440-1681.2008.05095.x
Ong HL, Ambudkar IS (2017) STIM-TRP pathways and microdomain organization: contribution of TRPC1 in store-operated Ca2+ entry impact on Ca2+ signaling and cell function. Adv Exp Med Biol 993:159–188. https://doi.org/10.1007/978-3-319-57732-6_9
Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW (2019) IP3 receptors and Ca2+ entry. Biochim Biophys Acta Mol Cell Res 1866(7):1092–1100. https://doi.org/10.1016/j.bbamcr.2018.11.007
Wu WKK, Zhang L, Chan MTV (2018) Autophagy, NAFLD and NAFLD-related HCC. Adv Exp Med Biol 1061:127–138. https://doi.org/10.1007/978-981-10-8684-7_10
Allaire M, Rautou PE, Codogno P, Lotersztajn S (2019) Autophagy in liver diseases: time for translation. J Hepatol 70(5):985–998. https://doi.org/10.1016/j.jhep.2019.01.026
Hazari Y, Bravo-San Pedro JM, Hetz C, Galluzzi L, Kroemer G (2020) Autophagy in hepatic adaptation to stress. J Hepatol 72(1):183–196. https://doi.org/10.1016/j.jhep.2019.08.026
Li S, Ning H, Ye Y, Wei W, Guo R, Song Q, Liu L, Liu Y, Na L, Niu Y, Chu X, Feng R, Moustaid-Moussa N, Li Y, Sun C (2017) Increasing extracellular Ca2+ sensitizes TNF-alpha-induced vascular cell adhesion molecule-1 (VCAM-1) via a TRPC1/ERK1/2/NFκB-dependent pathway in human vascular endothelial cells. Biochim Biophys Acta Mol Cell Res 1864(10):1566–1577. https://doi.org/10.1016/j.bbamcr.2017.06.001
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol 4(7):517–529. https://doi.org/10.1038/nrm1155
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35(6):600–604. https://doi.org/10.3109/10799893.2015.1030412
Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, Kroemer G (2013) Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol 23(5):310–322. https://doi.org/10.1016/j.semcancer.2013.05.008
Wang H, Liu Y, Wang D, Xu Y, Dong R, Yang Y, Lv Q, Chen X, Zhang Z (2019) The upstream pathway of mTOR-mediated autophagy in liver diseases. Cells 8(12):1597. https://doi.org/10.3390/cells8121597
Ye L, Cao Z, Lai X, Wang W, Guo Z, Yan L, Wang Y, Shi Y, Zhou N (2018) Niacin fine-tunes energy homeostasis through canonical GPRA signaling. FASEB J 33(4):4765–4779. https://doi.org/10.1096/fj.201801951R
Gastaldelli A (2017) Insulin resistance and reduced metabolic flexibility: cause or consequence of NAFLD? Clin Sci (Lond) 131(22):2701–2704. https://doi.org/10.1042/CS20170987
Zhang B, Li M, Zou Y, Guo H, Zhang B, Xia C, Zhang H, Yang W, Xu C (2019) NFκB/Orai1 facilitates endoplasmic reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Front Cell Dev Biol 7:202. https://doi.org/10.3389/fcell.2019.00202
Moccia F, Zuccolo E, Poletto V, Turin I, Guerra G, Pedrazzoli P, Rosti V, Porta C, Montagna D (2016) Targeting Stim and Orai proteins as an alternative approach in anticancer therapy. Curr Med Chem 23(30):3450–3480. https://doi.org/10.2174/0929867323666160607111220
Rychkov GY, Barritt GJ (2011) Expression and function of TRP channels in liver cells. Adv Exp Med Biol 704:667–686. https://doi.org/10.1007/978-94-007-0265-3_35
Feriod CN, Oliveira AG, Guerra MT, Nguyen L, Richards KM, Jurczak MJ, Ruan HB, Camporez JP, Yang X, Shulman GI, Bennett AM, Nathanson MH, Ehrlich BE (2017) Hepatic inositol 1,4,5 trisphosphate receptor type 1 mediates fatty liver. Hepatol Commun 1(1):23–35. https://doi.org/10.1002/hep4.1012
Tang M, Jiang Y, Jia H, Patpur BK, Yang B, Li J, Yang C (2020) Osteopontin acts as a negative regulator of autophagy accelerating lipid accumulation during the development of nonalcoholic fatty liver disease. Artif Cells Nanomed Biotechnol 48(1):159–168. https://doi.org/10.1080/21691401.2019.1699822
Xue W, Wang J, Jiang W, Shi C, Wang X, Huang Y, Hu C (2020) Caveolin-1 alleviates lipid accumulation in NAFLD associated with promoting autophagy by inhibiting the Akt/mTOR pathway. Eur J Pharmacol 871:172910. https://doi.org/10.1016/j.ejphar.2020.172910
Ren H, Wang D, Zhang L, Kang X, Li Y, Zhou X, Yuan G (2019) Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging (Albany NY) 11(21):9461–9477. https://doi.org/10.18632/aging.102396
Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL (2006) STIM1 has a plasma membrane role in the activation of store-operated Ca2+channels. Proc Natl Acad Sci USA 103(11):4040–4045. https://doi.org/10.1073/pnas.0510050103
Lopez JJ, Jardin I, Albarrán L, Sanchez-Collado J, Cantonero C, Salido GM, Smani T, Rosado JA (2020) Molecular basis and regulation of store-operated calcium entry. Adv Exp Med Biol 1131:445–469. https://doi.org/10.1007/978-3-030-12457-1_17
Bavencoffe A, Zhu MX, Tian JB (2017) New aspects of the contribution of ER to SOCE regulation: TRPC proteins as a link between plasma membrane ion transport and intracellular Ca2+ stores. Adv Exp Med Biol 993:239–255. https://doi.org/10.1007/978-3-319-57732-6_13
Ogasawara Y, Tsuji T, Fujimoto T (2020) Multifarious roles of lipid droplets in autophagy—target, product, and what else? Semin Cell Dev Biol 108:47–54. https://doi.org/10.1016/j.semcdb.2020.02.013
Khawar MB, Gao H, Li W (2019) Autophagy and lipid metabolism. Adv Exp Med Biol 1206:359–374. https://doi.org/10.1007/978-981-15-0602-4_17
Ge P, Wei L, Zhang M, Hu B, Wang K, Li Y, Liu S, Wang J, Li Y (2018) TRPC1/3/6 inhibition attenuates the TGF-β1-induced epithelial-mesenchymal transition in gastric cancer via the Ras/Raf1/ERK signaling pathway. Cell Biol Int 42(8):975–984. https://doi.org/10.1002/cbin.10963
Garaud S, Taher TE, Debant M, Burgos M, Melayah S, Berthou C, Parikh K, Pers JO, Luque-Paz D, Chiocchia G, Peppelenbosch M, Isenberg DA, Youinou P, Mignen O, Renaudineau Y, Mageed RA (2018) CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes. Cell Mol Immunol 15(2):158–170. https://doi.org/10.1038/cmi.2016.42
Zhong W, Chebolu S, Darmani NA (2019) Intracellular emetic signaling cascades by which the selective neurokinin type 1 receptor (NK1R) agonist GR73632 evokes vomiting in the least shrew (Cryptotis parva). Neurochem Int 122:106–119. https://doi.org/10.1016/j.neuint.2018.11.012
Vicencio JM, Estrada M, Galvis D, Bravo R, Contreras AE, Rotter D, Szabadkai G, Hill JA, Rothermel BA, Jaimovich E, Lavandero S (2011) Anabolic androgenic steroids and intracellular calcium signaling: a mini review on mechanisms and physiological implications. Mini Rev Med Chem 11(5):390–398. https://doi.org/10.2174/138955711795445880
Liu Q, Zhu P, Liu S, Tang M, Wang Y, Tian Y, Jin Z, Li D, Yan D (2019) NMAAP1 maintains M1 phenotype in macrophages through binding to IP3R and activating calcium-related signaling pathways. Protein Pept Lett 26(10):751–757. https://doi.org/10.2174/0929866526666190503105343
Li W, Zhang L (2019) Regulation of ATG and autophagy initiation. Adv Exp Med Biol 1206:41–65. https://doi.org/10.1007/978-981-15-0602-4_2
Noda NN, Inagaki F (2015) Mechanisms of autophagy. Annu Rev Biophys 44:101–122. https://doi.org/10.1146/annurev-biophys-060414-034248
Tanida I (2011) Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 14(11):2201–2214. https://doi.org/10.1089/ars.2010.3482
Acknowledgements
We thank Elsevier Language Editing Services for their linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by Department of Health of Zhejiang Province, China [Grant Number 2018KY553]; the Young and Middle-Aged Research Innovation Foundation of the Zhejiang Chinese Medical University, China [Grant Number KC201921]; and the Teacher Professional Development Project Foundation of University Visiting Scholars, China [Grant Number FX2019021].
Author information
Authors and Affiliations
Contributions
LZ and MW developed the idea and obtained the fund. LZ designed the study. LZ and YZ executed the experiments, analyzed the data, and drafted the manuscript. LZ and YJ wrote the manuscript. XD, SL, and HC provided experimental resources and critically evaluated the manuscript. QQ provided experimental resources and support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Zhang, Y., Jiang, Y. et al. Upregulated SOCC and IP3R calcium channels and subsequent elevated cytoplasmic calcium signaling promote nonalcoholic fatty liver disease by inhibiting autophagy. Mol Cell Biochem 476, 3163–3175 (2021). https://doi.org/10.1007/s11010-021-04150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04150-0