Abstract
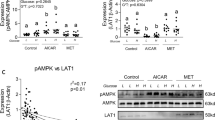
Population data have consistently demonstrated a correlation between circulating branched-chain amino acids (BCAA) and insulin resistance. Most recently valine catabolite, 3-hydroxyisobutyrate, has emerged as a potential cause of BCAA-mediated insulin resistance; however, it is unclear if valine independently promotes insulin resistance. It is also unclear if excess valine influences the ability of cells to degrade BCAA. Therefore, this study investigated the effect of valine on muscle insulin signaling and related metabolism in vitro. C2C12 myotubes were treated with varying concentrations (0.5 mM–2 mM) of valine for up to 48 h. qRT-PCR and western blot were used to measure metabolic gene and protein expression, respectively. Insulin sensitivity (indicated by pAkt:Akt), metabolic gene and protein expression, and cell metabolism were also measured following valine treatment both with and without varying levels of insulin resistance. Mitochondrial and glycolytic metabolism were measured via oxygen consumption and extracellular acidification rate, respectively. Valine did not alter regulators of mitochondrial biogenesis or glycolysis; however, valine reduced branched-chain alpha-keto acid dehydrogenase a (Bckdha) mRNA (but not protein) expression which was exacerbated by insulin resistance. Valine treatment had no effect on pAkt:Akt following either acute or 48-h treatment, regardless of insulin stimulation or varying levels of insulin resistance. In conclusion, despite consistent population data demonstrating a relationship between circulating BCAA (and related metabolites) and insulin resistance, valine does not appear to independently alter insulin sensitivity or worsen insulin resistance in the myotube model of skeletal muscle.








Similar content being viewed by others
Abbreviations
- 3HIB:
-
3-Hydroxyisobutyrate
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- BCAA:
-
Branched-chain amino acid
- BCAT:
-
Branched-chain aminotransferase
- BCKDH:
-
Branched-chain alpha-keto acid dehydrogenase
- BCKDK:
-
Branched-chain alpha-keto acid dehydrogenase kinase
- CS:
-
Citrate synthase
- ECAR:
-
Extracellular acidification rate
- FCCP:
-
Carbonyl cyanide p-[trifluoromethoxy]-phenyl-hydrazone
- GLUT4:
-
Glucose transporter 4
- HIBADH:
-
3-Hydroxyisobutyrate dehydrogenase
- LDHa:
-
Lactate dehydrogenase A
- NRF1/2:
-
Nuclear respiratory factor 1/2
- OCR:
-
Oxygen consumption rate
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha
- PK:
-
Pyruvate kinase
- Sirt1/3:
-
NAD+-dependent deacetylase sirtuin-1/3
- TBP:
-
TATA Binding Protein
- TFAM:
-
Mitochondrial transcription factor A
References
Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, Prehn C, Marette A, Adamski J, Tchernof A (2015) Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab 309:E736–E746. https://doi.org/10.1152/ajpendo.00231.2015
Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE (2009) Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32:1678–1683. https://doi.org/10.2337/dc08-2075
Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH (2013) Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 304:E1175–E1187. https://doi.org/10.1152/ajpendo.00630.2012
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10:723–736. https://doi.org/10.1038/nrendo.2014.171
Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, Lim SC, Khoo CM, Shah SH, Newgard CB (2010) Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53:757–767. https://doi.org/10.1007/s00125-009-1637-8
Lee CC, Watkins SM, Lorenzo C, Wagenknecht LE, Il'yasova D, Chen YD, Haffner SM, Hanley AJ (2016) Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 39:582–588. https://doi.org/10.2337/dc15-2284
Takashina C, Tsujino I, Watanabe T, Sakaue S, Ikeda D, Yamada A, Sato T, Ohira H, Otsuka Y, Oyama-Manabe N, Ito YM, Nishimura M (2016) Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr Metab 13:5. https://doi.org/10.1186/s12986-015-0059-5
Yamada C, Kondo M, Kishimoto N, Shibata T, Nagai Y, Imanishi T, Oroguchi T, Ishii N, Nishizaki Y (2015) Association between insulin resistance and plasma amino acid profile in non-diabetic Japanese subjects. J Diabetes Investig 6:408–415. https://doi.org/10.1111/jdi.12323
Tillin T, Hughes AD, Wang Q, Wurtz P, Ala-Korpela M, Sattar N, Forouhi NG, Godsland IF, Eastwood SV, McKeigue PM, Chaturvedi N (2015) Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 58:968–979. https://doi.org/10.1007/s00125-015-3517-8
Wurtz P, Makinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Ronnemaa T, Kahonen M, Lehtimaki T, Ripatti S, Raitakari OT, Jarvelin MR, Ala-Korpela M (2012) Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 61:1372–1380. https://doi.org/10.2337/db11-1355
Zhao X, Han Q, Liu Y, Sun C, Gang X, Wang G (2016) The Relationship between branched-chain amino acid related metabolomic signature and insulin resistance: a systematic review. J Diabetes Res 2016:2794591. https://doi.org/10.1155/2016/2794591
Gannon NP, Schnuck JK, Vaughan RA (2018) BCAA metabolism and insulin sensitivity—dysregulated by metabolic status? Mol Nutr Food Res 62:e1700756. https://doi.org/10.1002/mnfr.201700756
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326. https://doi.org/10.1016/j.cmet.2009.02.002
Newgard CB (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15:606–614. https://doi.org/10.1016/j.cmet.2012.01.024
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22:421–426. https://doi.org/10.1038/nm.4057
Miyazaki T, Honda A, Ikegami T, Iwamoto J, Monma T, Hirayama T, Saito Y, Yamashita K, Matsuzaki Y (2015) Simultaneous quantification of salivary 3-hydroxybutyrate, 3-hydroxyisobutyrate, 3-hydroxy-3-methylbutyrate, and 2-hydroxybutyrate as possible markers of amino acid and fatty acid catabolic pathways by LC-ESI-MS/MS. Springerplus 4:494. https://doi.org/10.1186/s40064-015-1304-0
Lyon ES, Rivera ME, Johnson MA, Sunderland KL, Vaughan RA (2019) Actions of chronic physiological 3-hydroxyisobuterate treatment on mitochondrial metabolism and insulin signaling in myotubes. Nutr Res 66:22–31. https://doi.org/10.1016/j.nutres.2019.03.012
Letto J, Brosnan ME, Brosnan JT (1986) Valine metabolism. Gluconeogenesis from 3-hydroxyisobutyrate. Biochem J 240:909–912. https://doi.org/10.1042/bj2400909
Liang C, Curry BJ, Brown PL, Zemel MB (2014) Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab 2014:239750–239750. https://doi.org/10.1155/2014/239750
Johnson MA, Gannon NP, Schnuck JK, Lyon ES, Sunderland KL, Vaughan RA (2018) Leucine, palmitate, or leucine/palmitate cotreatment enhances myotube lipid content and oxidative preference. Lipids 53:1043–1057. https://doi.org/10.1002/lipd.12126
Sato Y, Obeng KA, Yoshizawa F (2018) Acute oral administration of L-leucine upregulates slow-fiber- and mitochondria-related genes in skeletal muscle of rats. Nutr Res 57:36–44. https://doi.org/10.1016/j.nutres.2018.05.006
Doi M, Yamaoka I, Fukunaga T, Nakayama M (2003) Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun 312:1111–1117
Nishitani S, Takehana K, Fujitani S, Sonaka I (2005) Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol 288:G1292–G1300. https://doi.org/10.1152/ajpgi.00510.2003
Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H, Guo F (2014) Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63:841–850. https://doi.org/10.1016/j.metabol.2014.03.006
Brunetta HS, de Camargo CQ, Nunes EA (2018) Does L-leucine supplementation cause any effect on glucose homeostasis in rodent models of glucose intolerance? A systematic review. Amino Acids 50:1663–1678. https://doi.org/10.1007/s00726-018-2658-8
Rivera ME, Lyon ES, Johnson MA, Vaughan RA (2020) Leucine increases mitochondrial metabolism and lipid content without altering insulin signaling in myotubes. Biochimie 168:124–133. https://doi.org/10.1016/j.biochi.2019.10.017
Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, Chen M, Wynn RM, Wang J, Gui WJ, Qi X, Lusis AJ, Li Z, Wang W, Ning G, Yang X, Chuang DT, Wang Y, Sun H (2019) Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68:1730–1746. https://doi.org/10.2337/db18-0927
Hatazawa Y, Tadaishi M, Nagaike Y, Morita A, Ogawa Y, Ezaki O, Takai-Igarashi T, Kitaura Y, Shimomura Y, Kamei Y, Miura S (2014) PGC-1alpha-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 9:e91006. https://doi.org/10.1371/journal.pone.0091006
Hatazawa Y, Qian K, Gong DW, Kamei Y (2018) PGC-1alpha regulates alanine metabolism in muscle cells. PLoS ONE 13:e0190904. https://doi.org/10.1371/journal.pone.0190904
Matsumoto T, Nakamura K, Matsumoto H, Sakai R, Kuwahara T, Kadota Y, Kitaura Y, Sato J, Shimomura Y (2014) Bolus ingestion of individual branched-chain amino acids alters plasma amino acid profiles in young healthy men. Springerplus 3:35. https://doi.org/10.1186/2193-1801-3-35
Mardinoglu A, Gogg S, Lotta LA, Stancakova A, Nerstedt A, Boren J, Bluher M, Ferrannini E, Langenberg C, Wareham NJ, Laakso M, Smith U (2018) Elevated plasma levels of 3-hydroxyisobutyric acid are associated with incident type 2 diabetes. EBioMedicine 27:151–155. https://doi.org/10.1016/j.ebiom.2017.12.008
Yang SJ, Kwak SY, Jo G, Song TJ, Shin MJ (2018) Serum metabolite profile associated with incident type 2 diabetes in Koreans: findings from the Korean genome and epidemiology study. Sci Rep 8:8207. https://doi.org/10.1038/s41598-018-26320-9
Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI, Cottrell SE, Geiger G, Barnes ME, Wisinski JA, Fenske RJ, Matkowskyj KA, Kimple ME, Alexander CM, Merrins MJ, Lamming DW (2018) Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 596:623–645. https://doi.org/10.1113/jp275075
Yang X, Mei S, Wang X, Li X, Liu R, Ma Y, Hao L, Yao P, Liu L, Sun X, Gu H, Liu Z, Cao W (2013) Leucine facilitates insulin signaling through a Galphai protein-dependent signaling pathway in hepatocytes. J Biol Chem 288:9313–9320. https://doi.org/10.1074/jbc.M112.409409
Zeanandin G, Balage M, Schneider SM, Dupont J, Hebuterne X, Mothe-Satney I, Dardevet D (2012) Differential effect of long-term leucine supplementation on skeletal muscle and adipose tissue in old rats: an insulin signaling pathway approach. Age 34:371–387. https://doi.org/10.1007/s11357-011-9246-0
Avogaro A, Bier DM (1989) Contribution of 3-hydroxyisobutyrate to the measurement of 3-hydroxybutyrate in human plasma: comparison of enzymatic and gas-liquid chromatography-mass spectrometry assays in normal and in diabetic subjects. J Lipid Res 30:1811–1817
Haufe S, Engeli S, Kaminski J, Witt H, Rein D, Kamlage B, Utz W, Fuhrmann JC, Haas V, Mahler A, Schulz-Menger J, Luft FC, Boschmann M, Jordan J (2017) Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr Metab Cardiovasc Dis 27:858–864. https://doi.org/10.1016/j.numecd.2017.07.001
Acknowledgements
Support for this work was provided by the Department of Exercise Science within the Congdon School of Health Sciences. We would like also to thank the Department of Physical Therapy (Congdon School of Health Sciences) for the use of shared lab space and equipment. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Contributions
MER conducted experiments and was lead author for the manuscript. ESL and MAJ conducted experiments and assisted with manuscript preparation. KLS assisted with manuscript preparation. RAV conceived the study, conducted and oversaw experiments, performed all statistical analyses, and oversaw manuscript preparation. All authors have read and approved the final manuscript. Authors and contributors declare no conflict of interest. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Corresponding author
Ethics declarations
Conflict of interest
Authors and contributors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2020_3720_MOESM1_ESM.tif
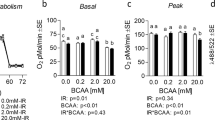
Fig. S1 Effect of valine at 2 mM for 48 on basal and peak mitochondrial and glycolytic metabolism. (a) Effect of valine at 2.0 mM for 48 h on myotube oxygen consumption (O2 pMol/min) under basal and peak metabolic conditions with Seahorse profile at right. (b) Effect of valine at 2.0 mM for 48 h on myotube glycolytic metabolism (mpH/min) under basal and peak metabolic conditions with Seahorse profile at right. Notes: Metabolism was analyzed using a student’s t test using n = 46 per group from two independent experiments. Oxygen consumption was normalized to non-mitochondrial respiration.Supplementary file1 (TIF 104 kb)
Rights and permissions
About this article
Cite this article
Rivera, M.E., Lyon, E.S., Johnson, M.A. et al. Effect of valine on myotube insulin sensitivity and metabolism with and without insulin resistance. Mol Cell Biochem 468, 169–183 (2020). https://doi.org/10.1007/s11010-020-03720-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03720-y