Abstract
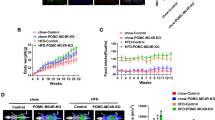
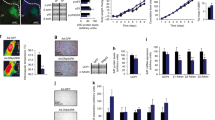
“With no lysine” (WNK) kinases have been shown to regulate various ion transporters in various tissues, but studies on the function of WNK kinases in the brain have been limited. In this study, we discovered that WNK1 and WNK4 in POMC-expressing neuronal cells in WNK1 overexpressed transgenic mice (WNK1 TG) decrease appetite via degradation of Kir6.2. Weight gain after 20 weeks of age was delayed in WNK1 TG mice as a result of reduced food intake. Expression of WNK1 and proopiomelanocortin (POMC) was higher in POMC-expressing neurons in the hypothalamus of WNK1 TG mice than in WT mice. Immunostaining of serial sections of the hypothalamus revealed that POMC-expressing neurons were smaller in WNK1 TG mice than in WT mice. In addition, expression of Kir6.2 was significantly reduced in WNK1 TG mice. Overexpression and knockdown of WNK4 demonstrated that WNK4 regulates protein expression of Kir6.2 via protein–protein interaction. Accordingly, reduced age-dependent weight gain of WNK1 TG mice seems to be related with the decreased Kir6.2 expression via WNK1- and WNK4-regulated protein stability of Kir6.2.





Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AgRP:
-
Agouti-related peptide
- ARC:
-
Arcuate nucleus
- ENaC:
-
Epithelial sodium channel
- KLHL3:
-
Kelch-like 3
- MC3R:
-
Melanocortin receptor 3
- MSH:
-
Melanocyte-stimulating hormone
- mTOR:
-
Mechanistic target of rapamycin
- NCC:
-
Na2+/Cl− cotransporter
- NPY:
-
Neuropeptide Y
- PHAII:
-
Pseudohypoaldosteronism type II
- POMC:
-
Proopiomelanocortin
- PTEN:
-
Phosphatase and tensin homolog
- WNK:
-
With no lysine
References
Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275:16795–16801
Verissimo F, Jordan P (2001) WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20:5562–5569. https://doi.org/10.1038/sj.onc.1204726
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112. https://doi.org/10.1126/science.1062844
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP (2006) Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38:1124–1132. https://doi.org/10.1038/ng1877
Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S (2009) Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet 18:3978–3986. https://doi.org/10.1093/hmg/ddp344
Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH (2010) Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem 285:25161–25167. https://doi.org/10.1074/jbc.M110.103432
Hadchouel J, Ellison DH, Gamba G (2016) Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78:367–389. https://doi.org/10.1146/annurev-physiol-021115-105431
Park HW, Kim JY, Choi SK, Lee YH, Zeng W, Kim KH, Muallem S, Lee MG (2011) Serine-threonine kinase with-no-lysine 4 (WNK4) controls blood pressure via transient receptor potential canonical 3 (TRPC3) in the vasculature. Proc Natl Acad Sci USA 108:10750–10755. https://doi.org/10.1073/pnas.1104271108
Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP (2004) WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc Natl Acad Sci USA 101:2064–2069. https://doi.org/10.1073/pnas.0308434100
Shekarabi M, Girard N, Riviere JB, Dion P, Houle M, Toulouse A, Lafreniere RG, Vercauteren F, Hince P, Laganiere J, Rochefort D, Faivre L, Samuels M, Rouleau GA (2008) Mutations in the nervous system—specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J Clin Invest 118:2496–2505. https://doi.org/10.1172/jci34088
Rinehart J, Vazquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP (2011) WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem 286:30171–30180. https://doi.org/10.1074/jbc.M111.222893
Roy A, Goodman JH, Begum G, Donnelly BF, Pittman G, Weinman EJ, Sun D, Subramanya AR (2015) Generation of WNK1 knockout cell lines by CRISPR/Cas-mediated genome editing. Am J Physiol Renal Physiol 308:F366–F376. https://doi.org/10.1152/ajprenal.00612.2014
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP (2013) Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA 110:7838–7843. https://doi.org/10.1073/pnas.1304592110
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP (2012) Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482:98–102. https://doi.org/10.1038/nature10814
Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, Sasaki S, Uchida S (2013) Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun 439:30–34. https://doi.org/10.1016/j.bbrc.2013.08.035
Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S (2014) Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet 23:5052–5060. https://doi.org/10.1093/hmg/ddu217
Wu G, Peng JB (2013) Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett 587:1717–1722. https://doi.org/10.1016/j.febslet.2013.04.032
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578. https://doi.org/10.1038/nn1455
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB (2007) Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228 – 32. https://doi.org/10.1038/nature06098
Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M (2013) Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33:3624–3632. https://doi.org/10.1523/jneurosci.2742-12.2013
Jo YH, Su Y, Gutierrez-Juarez R, Chua S Jr (2009) Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol 101:2305–2316. https://doi.org/10.1152/jn.91294.2008
Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S (2001) ATP-sensitive K + channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4:507–512. https://doi.org/10.1038/87455
Park YB, Choi YJ, Park SY, Kim JY, Kim SH, Song DK, Won KC, Kim YW (2011) ATP-sensitive potassium channel-deficient mice show hyperphagia but are resistant to obesity. Diabetes Metab J 35:219–225. https://doi.org/10.4093/dmj.2011.35.3.219
Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC (2006) Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116:1886–1901. https://doi.org/10.1172/jci27123
Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY (2012) Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 75:425–436. https://doi.org/10.1016/j.neuron.2012.03.043
Yang CL, Angell J, Mitchell R, Ellison DH (2003) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111:1039–1045. https://doi.org/10.1172/jci17443
Schumacher FR, Sorrell FJ, Alessi DR, Bullock AN, Kurz T (2014) Structural and biochemical characterization of the KLHL3-WNK kinase interaction important in blood pressure regulation. Biochem J 460:237–246. https://doi.org/10.1042/bj20140153
Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM (2007) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171:513–524. https://doi.org/10.2353/ajpath.2007.070188
Mori Y, Mori T, Wakabayashi M, Yoshizaki Y, Zeniya M, Sohara E, Rai T, Uchida S (2015) Involvement of selective autophagy mediated by p62/SQSTM1 in KLHL3-dependent WNK4 degradation. Biochem J 472:33–41. https://doi.org/10.1042/bj20150500
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) Grant, which was funded by the Korean Government (MEST) (NFR-2015R1D1A1A02062027 and NFR-2017R1A2B4010319).
Author information
Authors and Affiliations
Contributions
JYK conceived of and designed the experiments. WYC performed most of the experiments. JWH and HW helped to maintain the mice strain and participated the analysis of weight gain and food intake of mice. JYK, WYC analysed the data. MGL participated in design experiments and contributed reagents, materials and instruments. JYK wrote the manuscripts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chung, W.Y., Han, J.W., Heo, W. et al. Overexpression of WNK1 in POMC-expressing neurons reduces weigh gain via WNK4-mediated degradation of Kir6.2. Mol Cell Biochem 447, 165–174 (2018). https://doi.org/10.1007/s11010-018-3301-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3301-4