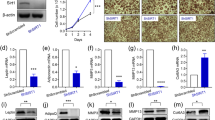
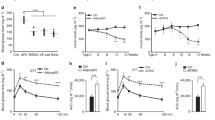
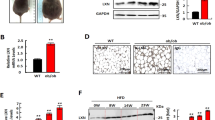
Abstract
Neprilysin (NEP) is a zinc metallopeptidase that cleaves a number of small peptides into inactive forms. Despite the recent evidence of a significant correlation between the levels of NEP in plasma and the severity of obesity in humans, a cause-and-effect relationship or a functional role of NEP in obesity has remained uncertain. In this study, we show that NEP has a positive regulatory effect on fat cell formation from precursor cells. NEP increases the accumulation of cytoplasmic triglycerides in 3T3-L1 preadipocytes or the C3H10T1/2 mesenchymal stem cell line in differentiation conditions. Consistently, cells expressing NEP showed an increase in mRNA expression of adipogenic transcription factors, peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), and the adipocyte markers aP2 and adipsin. Furthermore, this NEP-enhanced induction of adipogenesis was found to require the enzymatic activity of NEP, leading to augmentation of the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway. In summary, our results indicate that NEP accelerates adipogenesis through enhancement of insulin-mediated PI3K-Akt activation and imply a high therapeutic value of NEP in treating obesity and obesity-related disorders.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Akt:
-
Protein kinase B
- aP2:
-
Adipocyte protein 2
- C/EBPα:
-
CCAAT-enhancer-binding protein α
- DMI:
-
Dexamethasone, 1-methyl-3-isobutylxanthine and insulin
- HFD:
-
High-fat diet
- IRES:
-
Internal ribosome entry sequence
- MMPs:
-
Matrix metalloproteinases
- NEP:
-
Neprilysin, neutral endopeptidase, EC3.4.24.11
- PI3K:
-
Phosphatidylinositol 3-kinase
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- qRT-PCR:
-
Quantitative real-time PCR
- SD:
-
Standard chow diet
References
Hsu IR, Kim SP, Kabir M, Bergman RN (2007) Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr 86:867S–871S.
Kong Z-L (2007) Conjugated linoleic acid and weight control; from the biomedical immune viewpoint. In: Bagchi D, Preuss H (eds) Obesity: epidemiology, pathophysiology, and prevention. CRC Press, Boca Raton, FL, pp 383–399
Moreno-Navarrete JM, Fernández-Real JM (2012) Adipocyte differentiation. In: Symonds ME (ed) Adipose tissue biology. Springer, New York, pp 17–38
Hirsch J, Batchelor B (1976) Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 5:299–311
Turner AJ, Isaac RE, Coates D (2001) The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23:261–269
Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552
Miners JS, Baig S, Tayler H, Kehoe PG, Love S (2009) Neprilysin and insulin-degrading enzyme levels are increased in Alzheimer disease in relation to disease severity. J Neuropathol Exp Neurol 68:902–914
Miners JS, Morris S, Love S, Kehoe PG (2011) Accumulation of insoluble amyloid-beta in down’s syndrome is associated with increased BACE-1 and neprilysin activities. J Alzheimer’s Dis 23:101–108
Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ (2011) Neprilysin, obesity and the metabolic syndrome. Int J Obes 35:1031–1040
Jang J, Lee J, Kim ST, Lee KY, Cho JY, Kweon DH, Kwon ST, Koh YH, Kim S, Yoon K (2012) Polycation-mediated enhancement of retroviral transduction efficiency depends on target cell types and pseudotyped Env proteins: implication for gene transfer into neural stem cells. Neurochem Int 60:846–851
Marie-Claire C, Ruffet E, Tiraboschi G, Fournie-Zaluski MC (1998) Differences in transition state stabilization between thermolysin (EC 3.4.24.27) and neprilysin (EC 3.4.24.11). FEBS Lett 438:215–219
Green H, Meuth M (1974) An established pre-adipose cell line and its differentiation in culture. Cell 3:127–133
Russell TR, Ho R (1976) Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc Natl Acad Sci USA 73:4516–4520
Rubin CS, Hirsch A, Fung C, Rosen OM (1978) Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem 253:7570–7578
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC (2007) Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J 21:2185–2194
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Arcaro A, Wymann MP (1993) Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296(Pt 2):297–301
Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR (1998) PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Investig 101:22–32
Yang MS, Hong JS, Kim ST, Lee KY, Park KW, Kwon ST, Kweon DH, Koh YH, Gaiano N, Yoon K (2011) Among gamma-secretase substrates Notch1 alone is sufficient to block neurogenesis but does not confer self-renewal properties to neural stem cells. Biochem Biophys Res Commun 404:133–138
Reznikoff CA, Brankow DW, Heidelberger C (1973) Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33:3231–3238
Pinney DF, Emerson CPJ (1989) 10T1/2 cells: an in vitro model for molecular genetic analysis of mesodermal determination and differentiation. Environ Health Perspect 80:221–227
Rangwala SM, Lazar MA (2000) Transcriptional control of adipogenesis. Annu Rev Nutr 20:535–559
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y (1999) Novel modulator for endothelial adhesion molecules. Circulation 100:2473–2476
Cook KS, Groves DL, Min HY, Spiegelman BM (1985) A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc Natl Acad Sci USA 82:6480–6484
Yun S-M, Cho S-J, Song JC, Song SY, Jo SA, Jo C, Yoon K, Tanzi RE, Choi E-J, Koh YH (2013) SUMO1 modulates Aβ generation via BACE1 accumulation. Neurobiol Aging 34:650–662
Schling P, Schafer T (2002) Human adipose tissue cells keep tight control on the angiotensin II levels in their vicinity. J Biol Chem 277:48066–48075
Luchsinger JA, Tang M, Shea S, Mayeux R (2002) Caloric intake and the risk of Alzheimer disease. Arch Neurol 59:1258–1263
Profenno LA, Porsteinsson AP, Faraone SV (2010) Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67:505–512
Hildreth KL, Van Pelt RE, Schwartz RS (2012) Obesity, insulin resistance, and Alzheimer’s disease. Obesity (Silver Spring) 20:1549–1557
Iwase A, Ando H, Nagasaka T, Shibata D, Harata T, Shimomura Y, Goto M, Kikkawa F (2006) Neutral endopeptidase expressed by decidualized stromal cells suppresses akt phosphorylation and deoxyribonucleic acid synthesis induced by endothelin-1 in human endometrium. Endocrinology 147:5153–5159
Sumitomo M, Iwase A, Zheng R, Navarro D, Kaminetzky D, Shen R, Georgescu MM, Nanus DM (2004) Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell 5:67–78
Herath CB, Lubel JS, Jia Z, Velkoska E, Casley D, Brown L, Tikellis C, Burrell LM, Angus PW (2009) Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol 297:G98–G106
Than A, Leow MK, Chen P (2013) Control of adipogenesis by the autocrine interplays between angiotensin 1–7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J Biol Chem 288:15520–15531
Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M (2001) Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 50:2080–2086
Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR (2002) Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 51:1093–1101
Bauters D, Scroyen I, Van Hul M, Lijnen HR (2015) Gelatinase A (MMP-2) promotes murine adipogenesis. Biochim Biophys Acta 1850:1449–1456
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (#2015R1A2A2A01005687).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial/commercial conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, J., Han, D., Byun, SH. et al. Neprilysin facilitates adipogenesis through potentiation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Mol Cell Biochem 430, 1–9 (2017). https://doi.org/10.1007/s11010-017-2948-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-2948-6