Abstract
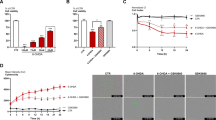
Parkinson’s disease (PD) can degenerate dopaminergic (DA) neurons in midbrain, substantia-nigra pars compacta. Alleviation of its symptoms and protection of normal neurons against degeneration are the main aspects of researches to establish novel therapeutic strategies. PPARγ as a member of PPARs have shown neuroprotection in a number of neurodegenerative disorders such as Alzheimer’s disease and PD. Nuclear receptor related 1 protein (Nurr1) is, respectively, member of NR4A family and has received great attentions as potential target for development, maintenance, and survival of DA neurons. Based on neuroprotective effects of PPARγ and dual role of Nurr1 in anti-inflammatory pathways and development of DA neurons, we hypothesize that PPARγ and Nurr1 agonists alone and in combined form can be targets for neuroprotective therapeutic development for PD in vitro model. 1-Methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in PC12 cells as an in vitro model for PD studies. Treatment/cotreatment with PPARγ and Nurr1 agonists 24 h prior to MPP+ induction enhanced the viability of PC12 cell. The viability of PC12 cells was determined by MTS test. Mitochondrial membrane potential (MMP) and intracellular reactive oxygen species (ROS) were detected by flow cytometry. In addition, the relative expression of four genes including TH (the marker of DA neurons), Ephrin A1, Nurr1, and Ferritin light chain were assessed by RT-qPCR. In the MPP+-pretreated PC12 cells, PPARγ and Nurr1 agonists and their combined form resulted in a decrease in the cell death rate. Moreover, production of intracellular ROS and MMP modulated by MPP+ was decreased by PPARγ and Nurr1 agonists’ treatment alone and in the combined form.







Similar content being viewed by others
Abbreviations
- 6-MP:
-
6-Mercaptopurine
- AD:
-
Alzheimer’s disease
- CREB:
-
cAMP response element binding
- DA:
-
Dopaminergic
- DAPI:
-
4′, 6-Diamidino-2-phenylindole
- DCF:
-
2′, 7′-Dichlorofluorescin
- DCFH2-DA:
-
6-Carboxy-2′, 7′-dichlorodihydrofluorescein diacetate
- DMSO:
-
Dimethyl sulfoxide
- DMEM:
-
Dulbecco’s modified eagle medium
- ETS:
-
Electron transfer system
- FITC:
-
Fluoroisothiocyanate
- FTL:
-
Ferritin light chain
- JC-1:
-
J-aggregate-forming fluorescent dye, 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolocarbocyanine
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- IL-1β:
-
Interleukin 1 beta
- LBD:
-
Lewy body dementia
- MMP:
-
Mitochondrial membrane potential
- MPP+ :
-
1-Methyl-4-phenylpyridinium ion
- MPTP:
-
1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- NGF:
-
Nerve growth factor
- PD:
-
Parkinson’s disease
- PGC1-α:
-
PPARγ coactivator 1α
- PMS:
-
Phenazine methosulfate
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- ROS:
-
Reactive oxygen species
- SNpc:
-
Substantia-nigra pars compacta
- SVZ:
-
Subventricular zone
- TBBPA:
-
Tetrabromobisphenol A (2,2-bis(4-hydroxy-3,5-dibromophenyl)propane
- TH:
-
Tyrosine hydroxylase
- TNF-α:
-
Tumor necrosis alpha
- TRITC:
-
Tetramethyl rhodamine-isothiocyanate
References
De Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet neurol 5:525–535
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53:S26–S36
Jodeiri Farshbaf M, Ghaedi K, Megraw TL, Curtiss J, Shirani Faradonbeh M, Vaziri P et al (2016) Does PGC1α/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative disorders? Neuromol Med 18(1):1–15
Schulz JB, Falkenburger BH (2004) Neuronal pathology in Parkinson’s disease. Cell Tissue Res 318(1):135–147
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20(5):649–688
Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169(3):453–459
Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE (2000) Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci 20:558–567
Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P et al (2008) Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28(37):9287–9296
Wojtowicz AK, Szychowski KA, Kajta M (2014) PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotox Res 5:311–322
Kaundal RK, Sharma SS (2011) Ameliorative effects of GW1929, a nonthiazolidinedione PPARγ agonist, on inflammation and apoptosis in focal cerebral ischemic-reperfusion injury. Curr Neurovasc Res 8:236–245
Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2004) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem 88:494–501
Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V et al (2005) Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol 70:177–188
Kim CH, Han BS, Moon J, Kim DJ, Shin J, Rajan S et al (2015) Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci USA 112:8756–8761
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons in Parkinson’s disease. Prog Neurobiol 77:128–138
Ordentlich P, Yan Y, Zhou S, Heyman R (2003) An identification of the antineoplastic agent 6-mercaptopurine as an activator of the orphan nuclear hormone receptor Nurr1. J Biol Chem 278:24791–24799
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG et al (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Shi Y (2007) Orphan nuclear receptors in drug discovery. Drug Discov Today 12(11–12):440–445
Yan J, Sun J, Huang L, Fu Q, Du G (2014) Simvastatin prevents neuroinflammation by inhibiting N-methyl-d-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J Neurosci Res 92:634–640
Jing X, Miwa H, Sawada T, Nakanishi I, Kondo T, Miyajima M et al (2012) Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One 7:e32019
Men T, Piao SH, Teng CB (2013) Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP. Yi Chuan 35:1283–1290
Parkinson GM, Dayas CV, Smith DW (2015) Age-related gene expression changes in substantia nigra dopamine neurons of the rat. Mech Ageing Dev 149:41–49
Praticó D, Pasin M, Barry OP, Ghiselli A, Sabatino G, Iuliano L et al (1999) Iron-dependent human platelet activation and hydroxyl radical formation: involvement of protein kinase C. Circulation 99(24):3118–3124
Dusek P, Schneider SA, Aaseth J (2016) Iron chelation in the treatment of neurodegenerative diseases. J Trace Elem Med Biol. doi:10.1016/j.jtemb.2016.03.010
Arosio P, Levi S (2010) Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta 1800(8):783–792
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203
Vidal R, Ghetti B, Takao M, Brefel-Courbon C, Uro-Coste E, Glazier BS et al (2004) Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol 63(4):363–380
Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L et al (2001) EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105:233–244
Gerlai R (2001) Eph receptors and neural plasticity. Nat Rev Neurosci 2(3):205–209
Aoki M, Yamashita T, Tohyama M (2004) EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J Biol Chem 279:32643–32650
Jing X, Miwa H, Sawada T, Nakanishi I, Kondo T, Miyajima M et al (2012) Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One 7(2):e32019
Wang AG, Chen CH, Yang CW, Yen MY, Hsu WM, Liu JH et al (2002) Change of gene expression profiles in the retina following optic nerve injury. Brain Res Mol Brain Res 101(1–2):82–92
Zhang Z, Li X, Xie WJ, Tuo H, Hintermann S, Jankovic J et al (2012) Anti-parkinsonian effects of Nurr1 activator in ubiquitin–proteasome system impairment induced animal model of Parkinson’s disease. CNS Neurol Disord Drug Targets 11:768–773
Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ et al (2003) Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem 85:622–634
Huot P, Lévesque M, Morissette M, Calon F, Dridi M, Di Paolo T et al (2008) L-Dopa treatment abolishes the numerical increase in striatal dopaminergic neurons in parkinsonian monkeys. J Chem Neuroanat 35(1):77–84
Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S (2010) A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci 30:963–972
Ghoochani A, Shabani K, Peymani M, Ghaedi K, Karamali F, Karbalaei K et al (2012) The influence of peroxisome proliferator-activated receptor γ1 during differentiation of mouse embryonic stem cells to neural cells. Differentiation 83:60–67
Peymani M, Ghoochani A, Ghaedi K, Karamali F, Karbalaie K, Kiani-Esfahani A et al (2013) Dual effects of peroxisome proliferator-activated receptor γ on embryonic stem cell self-renewal in presence and absence of leukemia inhibitory factor. Eur J Cell Biol 92:160–168
Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK et al (2008) Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 28:60–67
Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J et al (2010) NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci USA 107:12317–12322
Fu AK, Ip NY (2007) Cyclin-dependent kinase 5 links extracellular cues to actin cytoskeleton during dendritic spine development. Cell Adhes Migr 1:110–112
Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N et al (2008) The PPARγ agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br J Pharmacol 154:226–233
Smith GA, Rocha EM, Rooney T, Barneoud P, McLean JR, Beagan J et al (2015) A Nurr1 agonist causes neuroprotection in a Parkinson’s disease lesion model primed with the toll-like receptor 3 dsRNA inflammatory stimulant poly(I:C). PLoS One 10:e0121072
Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW et al (2009) PPARγ inhibits NF-κB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metabol 297:174–183
Dubois C, Hengerer B, Mattes H (2006) Identification of a potent agonist of the orphan nuclear receptor Nurr1. Chem Med Chem 1:955–958
De Miranda BR, Popichak KA, Hammond SL, Miller JA, Safe S, Tjalkens RB (2015) Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson’s disease. Toxicol Sci 143:360–373
Lou X, Liao W (2012) Association of Nurr1 gene mutations with Parkinson’s disease in the Han population living in the Hubei province of China. Neural Regen Res 7(23):1791–1796
Esteves M, Cristóvão AC, Saraiva T, Rocha SM, Baltazar G, Ferreira L et al (2015) Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front Aging Neurosci 7:20
Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF et al (2001) Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet 28(4):350–354
Friedman A, Arosio P, Finazzi D, Koziorowski D, Galazka-Friedman J (2011) Ferritin as an important player in neurodegeneration. Parkinsonism Relat Disord 17:423–430
Mounsey RB, Teismann P (2012) Chelators in the treatment of iron accumulation in Parkinson’s disease. Int J Cell Biol 2012:983245
Li X, Jankovic J, Le W (2011) Iron chelation and neuroprotection in neurodegenerative diseases. J Neural Transm (Vienna) 118(3):473–477
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Lonze BE, Riccio A, Cohen S, Ginty DD (2002) Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34:371–385
Wilkinson DG (2001) Multiple roles of Eph receptors and ephrins in neural development. Nat Rev Neurosci 2:155–164
Irani K, Goldschmidt-Clermont PJ (1998) Ras, superoxide and signal transduction. Biochem Pharmacol 55:1339–1346
Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ et al (1988) Terada and LS Xanthine oxidase derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Invest 81:1556–1562
Susin SA, Zamzami N, Kroemer G (1996) The cell biology of apoptosis: evidence for the implication of mitochondria. Apoptosis 1:231–242
Troy CM, Salvesen GS (2002) Caspases on the brain. J Neurosci Res 69:145–150
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm 1(1):14
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediat Inflamm 2013:342931
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47
Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S et al (2000) Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm (Vienna) 107(3):335–341
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM et al (2007) Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 104(47):18754–18759
Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-κB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol 7(3):544–556
Klesse LJ, Meyers KA, Marshall CJ, Parada LF (1999) Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene 18:2055–2068
Acknowledgments
This study was funded by a grant-in-aid of research from Royan Institute awarded to Kamran Ghaedi, Ph.D. as the Principal Investigator (P.I.), and in support of Mohammad Jodeiri Farshbaf for obtaining his M.Sc. degree from the University of Isfahan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interest to disclose and all authors support submission to this journal.
Ethical improvement statement
Approval for this study was obtained from the Institutional Review Board of Royan Institute (Tehran, Iran).
Additional information
Mohammad Jodeiri Farshbaf and Mahboobeh Forouzanfar contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2764_MOESM1_ESM.tif
Supplementary Figure 1: Cell proliferation modulation by GW1929, 6-MP. PC12 cells were cultured in the presence different amounts of GW1929, 6-MP. Results were expressed as percentages of viable cells in relation to the control samples (Vehicle only). Represented values are the mean of duplicate independent experiments. (TIFF 155 kb)
11010_2016_2764_MOESM2_ESM.tif
Supplementary Figure 2: Apoptosis rate was decreased by pretreatment of the cells with GW1929, 6-MP. The number of annexin V positive cells was significantly decreased when GW1929, 6-MP and their combination were used. Results were expressed as the percentage of cell number after treating with MPP+. Represented values are the mean of triplicate independent experiments ±SEM. Similar alphabets indicate significant difference between same samples at p<0.05. (TIFF 151 kb)
11010_2016_2764_MOESM3_ESM.tif
Supplementary Figure 3: Quantification of PPARγ and Nurr1 intracellular distribution upon activation. Intracellular localization of PPARγ and Nurr1 in cells indicated predominantly nuclear-sorting [72% nuclear distribution vs. 28% cytosolic distribution for PPARγ and 85% nuclear distribution vs. 15% cytosolic distribution for Nurr1] after activation by GW1929 (10 μM) and 6-MP (0.5 µM), respectively, as compared with the untreated samples (-GW1929 and -6-MP) [14% nuclear distribution vs. 86% cytosolic distribution for PPARγ and 23% nuclear distribution vs. 77% cytosolic distribution for Nurr1]. (TIFF 151 kb)
Rights and permissions
About this article
Cite this article
Jodeiri Farshbaf, M., Forouzanfar, M., Ghaedi, K. et al. Nurr1 and PPARγ protect PC12 cells against MPP+ toxicity: involvement of selective genes, anti-inflammatory, ROS generation, and antimitochondrial impairment. Mol Cell Biochem 420, 29–42 (2016). https://doi.org/10.1007/s11010-016-2764-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2764-4