Abstract
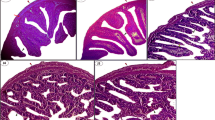
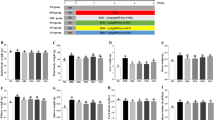
Marine fishes are important to health due to their high content of polyunsaturated fatty acids particularly those of the omega-3 family. These fatty acids play an important role in various physiological processes and as a consequence they may modulate and even prevent some human diseases. The aim of the present study was to investigate and compare the effect of fish oils of different origins (Sardinella longiceps, Rastrelliger kanagurta and Clarias batrachus) on lipid metabolism and membrane fluidity in diabetes. Alloxan was injected in repetitive doses for 1 month (100 mg/kg body weight every 5th day) to induce diabetes in Swiss albino mice. 10 % S. longiceps, R. kanagurta or C. batrachus fish oil was freshly blended with pellet feed which was provided to diabetic mice for 1 month. The serum lipid profile (serum total cholesterol, triglyceride, HDL, VLDL and LDL) along with liver, kidney and heart tissue lipid profile (i.e. triglyceride, total cholesterol, glycolipid and phospholipid) was analysed. Besides, the enzymatic activity of HMG-CoA reductase, HMG-CoA synthase and glucose-6-phosphate-dehydrogenase along with the membrane fluidity of these tissues was evaluated. Altered tissue lipid composition, enzyme activities and membrane fluidity due to diabetes were returned towards normal with the supplementation of 10 % fish oils. Fish oil from S. longiceps brought maximum changes in level of neutral lipid composition in heart, and increased the concentration of phospholipid and decreased the activity of HMG-CoA reductase in comparison with the fish oil from R. kanagurta and C. batrachus.





Similar content being viewed by others
Abbreviations
- HDL-C:
-
High-density lipoprotein cholesterol
- VLDL-C:
-
Very low-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglyceride
- MG:
-
Mono-glyceride
- DG:
-
Di-glyceride
- FFA:
-
Free fatty acid
- PL:
-
Phospholipid
- TC:
-
Total cholesterol
- CE:
-
Cholesteryl esters
- HMG-CoA reductase:
-
3-Hydroxy-3-methyl-glutaryl-CoA reductase
- HMG-CoA synthase:
-
3-Hydroxy-3-methyl-glutaryl-CoA synthase
- G6PD:
-
Glucose-6-phosphate-dehydrogenase
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- PUFA:
-
Polyunsaturated fatty acid
- DM:
-
Diabetes mellitus
References
Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR (1999) Diabetes and cardiovascular disease a statement for healthcare professionals from the American Heart Association. Circulation 100:1134–1146. doi:10.1161/01.CIR.100.10.1134
Gennis RB (1989) Biomembranes: molecular structure and function. Springer, Berlin. ISBN 0387967605
Goldberg IJ (2001) Diabetic dyslipidaemia: causes and consequences. J Clin Endocrinol Metab 86(3):966–971
Feitosa ACR, Feitosa-Filho GS, Freitas FR, Wajchenberg BL, Maranhão RC (2013) Lipoprotein metabolism in patients with type 1 diabetes under intensive insulin treatment. Lipids Health Dis 12(15):1–8
Balasubramanyam A (2015) Diabetic dyslipidaemia. http://www.medscape.org/viewarticle/418584
Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, D’ Agostino R, Marcovina S, Dabelea D (2009) Lipid and lipoprotein profiles in youth with and without type 1 diabetes. Diabetes Care 32(3):416–420. doi:10.2337/dc08-1775
Simpoulous AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70(3):560s–569s
Zamaria N (2004) Alteration of PUFA status and metabolism in health and disease. Reprod Nutr Dev 44(3):273–282
Ruxton CSH, Reed SC, Simpson MJA, Millington KJ (2004) The health benefits of omega-3 polyunsaturated fatty acid: a review of the evidence. J Hum Nutr Diet 17:449–459
Siriwardhana N, Kalupahana NS, Moustaid-Moussa N (2012) Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and Docosahexaenoic acid. Adv Food Nutr Res 65:211–222. doi:10.1016/B978-0-12-416003-3.00013-5
Brownstein J (2014) Fish oil supplements: Sound science or mostly hype. Livescience. http://www.livescience.com/46635-fish-oil-supplements-hype.html. Accessed 02 July 2014
Tsuduki T, Honna T, Nakagawa K, Ikeda I, Miyazawa T (2011) Long term intake of fish oil increases oxidative stress and decreases lifespan in senescence- accelerated mice. Nutrition 27:334–337
Folch J, Less S, Stainley GH (1957) A simple method for isolation and purification of total lipids from animal tissue. J Biol Chem 226:497–509
Anstall HB, Trujillo JM (1965) Determination of free fatty acids in plasma by a colorimetric procedure: an appraisal of the method and comparison with other techniques. Clin Chem 11:741–747
Kates M (1986) Techniques of lipidology, isolation, analysis and identification of lipids. Elsevier, New York
Carroll NV, Longley RW, Roe JH (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem 220:583–593
O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT (2007) A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci 85:1511–1521. doi:10.2527/jas.2006-491
Kleinfeld AM, Dragsten P, Klausner RD, Pjura WJ, Matayoshi ED (1981) The lack of relationship between fluorescence polarization and lateral diffusion in biological membranes. Biochim Biophys Acta 649(2):471–480
Siedel J (1983) 3-hydroxy-3methylglutaryl-CoA reductase. Mehods of enzymatic analysis, vol 111, 3rd edn. Verlag Chemie GmbH, Weinheim, pp 156–161
Miziorko HM (1985) 3-hydroxy-3methylglutaryl-CoA synthase. In: Colowick CO, Kalpana N (eds) Methods in enzymology, vol 110. Academic Press, New York, pp 19–26
Anderson W, Nordlie R (1968) Worthington Biochemical Corporation: glucose-6-phosphate dehydrogenase assay manual: glucose dehydrogenase activity of yeast glucose-6-dehydrogenase. I. selective stimulation by bicarbonate, phosphate, and sulfate. Biochemistry 7:1479–1485
Kamat SG, Roy R (2015) Evaluation of fish oils in amelioration of diabetes-induced tissue damages in mice (Mus musculus). South Asia J Expt Biol 5(1):32–40
Kamat SG, Roy R (2015) Evaluation of antioxidant potential of Clarias Batrachus oil in alloxan induced diabetic mice (Mus Musculus). J Diabetes Metab 6:552. doi:10.4172/2155-6156.1000552
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J (2011) The role of oxidative stress and antioxidants in diabetic complications. SQU Med J 12:5–18
Donath MY, Storling J, Maedler K, Mandrup-Poulsen T (2003) Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 81(8):455–470
Kromhout D, Bosschieter EB, Coulander CDL (1985) The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 312:1205–1209
Kris-Etherton PM, Harris WS, Appel LJ, Burr ML, Gilbert JF (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757. doi:10.1161/01.CIR0000038493.65177.94
Burr ML, Gilbert JF, Hollliday RM, Sweetnam PM, Elwood PC, Deadman NM (1989) Effects of changes of fat, fish, and fibre intakes on death from myocardial reinfarction: diet and Reinfarction Trial (DART). Lancet 2:757–761
Mathew KKAS, Lakshmanan PT (2012) Effect of PUFA from fish oil on lipid metabolism of heart in streptozotocin induced diabetes in male albino mice. J Biomed Sci 1(1):1–7
Yates A, Norwig J, Marron JC, Bost J, Bradley JP, Duca M, Wecht DA, Grove R, Iso A, Cobb I, Ross N, Borden M (2009) Evaluation of lipid profiles and use of omega-3 essential fatty acids in professional football players. Sports Health 1(1):21–30
Sharma N, Garg V (2009) Antidiabetic and antioxidant potential of ethanolic extract from Butea monosperma leaves in alloxan-induced diabetic mice. Indian J Biochem Biophys 46:99–105
Qi XY, Chen WJ, Zhang LQ, Xie BJ (2008) Mogrosides extract from Siraitia grosvenorii scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr Res 28(4):278–284. doi:10.1016/j.nutres.2008.02.008
Verges B (2001) Insulin sensitivity and lipids. Diabetes Metab 27(2pt2):223–227
Ginsberg HN (1996) Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes 45(3):S27–S30
Young NL, Saudek CD, Crawford SA (1982) Total hydroxymethylglutaryl CoA reductase activity in the small intestine and liver of insulin-deficient rats. J Lipid Res 23:266–275
Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M (2005) Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54:2328–2335
Oni GA, Johnson RA, Oguntibeju OO (2005) Detecting patients with glucose-6-phosphate-dehydrogenase deficiency. JIACM 6:42–44
Zhang Z, Apse K, Pang J, Stanton RC (2010) High glucose inhibits glucose-6-phosphate-dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J 24(5):1497–1505. doi:10.1096/fj.09-136572
Xu Y, Osborne B, Stanton RC (2005) Diabetes causes inhibition of glucose-6-phosphate-dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 289:1040–1047
Diaz-Flores M, Ibanez-Harnandez MA, Galvan RE, Gutierrez M, Duran-Reyes G, Median-Navarro R, Pasceo-Lira D, Ortega-Camarillo C, Vilar-Rojas C, Cruz M, Baize-Gutman LA (2006) Glucose-6-phosphate-dehydrogenase activity and NADPH/NADP ratio in liver and pancreas are dependent on the severity of hyperglycaemia in rat. Life Sci 78:2601–2607
Choy PC, Hatch GM, Man RYK (1997) Phospholipid biosynthesis in health and disease. Adv lipobiol 2:47–78
Novgorodtseva TP, Karaman YK, Zhukova NV, Lobanova EG, Antonyuk MV, Kantur TA (2011) Composition of fatty acids in plasma and erythrocytes and eicosanoids level in patients with metabolic syndrome. Lipids Health Dis 10:82. doi:10.1186/1476-511X-10-82
Waczulíková I, Šikurová L, Čársky J, Štrbová L, Krahulec B (2000) Decreased fluidity of isolated erythrocyte membranes in type 1 and tpe 2 diabetes. The effect of resorcylidene aminoguanidine. Gen Physiol Biophys 19(4):381–392
Bryszewska M, Watala C, Torzecka W (1986) Changes in fluidity and composition of erythrocyte membranes and in composition of plasma lipids in type I diabetes. Br J Haematol 62:111–116. doi:10.1111/j.1365-2141.1986.tb02906.x
Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O (1995) Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Diabetes Care 18:549–551
Watala C, Kordacka M, Loba A, Jόzwiak Z, Nowak S (1987) Analysis of membrane fluidity alterations and lipid disorders in type I diabetic children and adolescents. Acta Diabetol Lat 24:141–148
Fox TE, Han X, Kelly S, Merrill AH, Marti RE, Aderson RE, Gardner TW, Kester M (2006) Diabetes alters sphingolipid mechanism in retina. Diabetes 55(12):3573–3580
Abott SK, Else PL, Atkins TA (1818) Hulbert AJ (2012) Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. BBA-Biomembr 5:1309–1317
Strolien HH, Kriketos AD, Calvert GD, Baur LA, Jenkins AB (2007) Fatty acids, triglycerides and syndromes of insulin resistance. Prostaglandins and plasma lipid and lipoprotein metabolism in human: a critical review. Diabetes Care 30(4):1012–1024
Soltan MAS (2012) The effects of varieties sources of omega-3 fatty acids on diabetes in rats. FNS 3:1404–1412. doi:10.4236/fns.2012.310184
Nestel PJ (1990) Effects of n23 fatty acids on lipid metabolism. Annu Rev Nutr 10:149–167
Nestel PJ (2000) Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr 7(1):228S–231S
El-Sohemy A, Archer MC (1999) Regulation of mevalonate synthesis in low density lipoprotein receptor knockout mice fed n-3 or n-6 polyunsaturated fatty acids. Lipids 34(10):1037–1043
Simopoulos AP, Cleland LG (2003) Omega-6/omega-3 essential fatty acid ratio: the scientific evidence. Basel Karger Medical and Scientific Publishers, Basel. doi:10.1159/000073789
Matesanz N, ParkG McAllister H, Leahey W, Devine A, McVeigh GE, Gardiner TA, McDonald DM (2010) Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Invest Ophthalmol Vis Sci 51(12):6815–6825. doi:10.1167/iovs.10-5339
Calder PC (2012) Mechanism of action of n-3 fatty acids. J Nutr 142(3):592S–599S. doi:10.3945/jn.111.155259
Acknowledgments
Financial support from the University Grant Commission-Special Assistance Programme India (Sanction letter no. F.3-3/2011 (SAPII) is acknowledged. We thank Professor Philip Calder, University of Southampton, for assistance with writing and for comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The study was carried out in strict accordance as per the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals, Govt. of India). The authors declare that they have no conflicts of interest concerning this article.
Rights and permissions
About this article
Cite this article
Kamat, S.G., Roy, R. Evaluation of the effect of n-3 PUFA-rich dietary fish oils on lipid profile and membrane fluidity in alloxan-induced diabetic mice (Mus musculus). Mol Cell Biochem 416, 117–129 (2016). https://doi.org/10.1007/s11010-016-2701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2701-6