Abstract
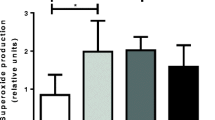
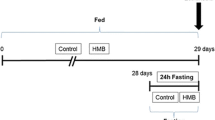
Myosin heavy chain (MHC) mediates the metabolic and contractile responses of skeletal muscles. MHC displays different isoforms, each of which has different characteristics. To better understand the effect of polyunsaturated fatty acids in skeletal muscles, rats were fed with control-, docosahexaenoic acid (DHA)-, and arachidonic acid (ARA)-oil, and the effects on plasma and muscular fatty acid profile, oxidative stress, mRNA levels of myosin heavy chain isoforms MHC1 of slow-twitch muscle (SO) and MHC2A, MHC2X, and MHCB isoforms of extensor digitorum longus (EDL) of fast-twitch muscle were evaluated. Concomitantly, mRNA levels of anti-oxidative enzymes, such as, catalase, glutathione peroxidase (GPx) and superoxide dismutase (SOD were determined. The expressions of MHC1, MHC2A, MHC2X, and MHC2B were lower in the SO of the DHA-fed rats. In the EDL muscles of DHA-fed rats, the expressions of MHC1 and MHC2A increased; however, the expressions of MHC2X increased and that of the MHC2 were not altered. Oxidative stress, as indicated by the levels of LPO, was significantly higher in the plasma of the ARA-fed rats, when compared with that of the DHA-fed rats. The LPO levels were higher both in the SO and EDL muscles of ARA-fed rats. Compared with ARA oil intake, DHA oil showed higher mRNA levels of GPx and SOD. Catalase expression was higher only in the EDL but not in the SO-type muscles. Our studies finally indicate that DHA and ARA differentially affect the regulation of contractile and metabolic properties of slow- and fast-twitch skeletal muscles.




Similar content being viewed by others
References
Bottinelli R (2001) Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch 443:6–17
Fitts RH, Widrick JJ (1996) Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24:427–473
Zierath JR, Hawley JA (2004) Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol 2:e348. doi:10.1371/journal.pbio.0020348
Pette D (1998) Training effects on the contractile apparatus. Acta Physiol Scand 162:367–376
Sato Y, Shimizu M, Mizunoya W, Wariishi H, Tatsumi R et al (2009) Differential expression of sarcoplasmic and myofibrillar proteins of rat soleus muscle during denervation atrophy. Biosci Biotechnol Biochem 73:1748–1756
Caiozzo VJ, Baker MJ, McCue SA, Baldwin KM (1997) Single-fiber and whole muscle analyses of MHC isoform plasticity: interaction between T3 and unloading. Am J Physiol 273:C944–C952
Yoshihara H, Wakamatsu J, Kawabata F, Mori S, Haruno A et al (2006) Beef extract supplementation increases leg muscle mass and modifies skeletal muscle fiber types in rats. J Nutr Sci Vitaminol (Tokyo) 52:183–193
Abrahao AC, Castilho RM, Squarize CH, Molinolo AA, dos Santos-Pinto D Jr, Gutkind JS (2010) A role for COX2-derived PGE2 and PGE2-receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol 46:880–887
Giudetti AM, Cagnazzo R (2012) Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat 99:57–67
Azain MJ (2004) Role of fatty acids in adipocyte growth and development. J Anim Sci 82:916–924
Gotoh N, Nagao K, Onoda S, Shirouchi B, Furuya K et al (2009) Effects of three different highly purified n-3 series highly unsaturated fatty acids on lipid metabolism in C57BL/KsJ-db/db mice. J Agric Food Chem 57:11047–11054
Baillie RA, Takada R, Nakamura M, Clarke SD (1999) Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids 60:351–356
Kam PC, See A (2000) Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia 55:442–449
Katori M, Majima M (2000) Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm Res 49:367–392
Kinsella JE, Broughton KS, Whelan JW (1990) Dietary unsaturated fatty acids: interactions and possible needs in relation to eicosanoid synthesis. J Nutr Biochem 1:123–141
Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ (2001) Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J Clin Endocrinol Metab 86:5067–5070
Markworth JF, Cameron-Smith D (2013) Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am J Physiol Cell Physiol 304:56–67
Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron M, Combaret L, Dardevet D (2009) Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587:5483–5492
Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ (2011) Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300:R655
Mizunoy W, Iwamoto Y, Shirouchi B, Sato M, Komiya Y, Razin RF, Ryuichi Tatsumi R, Sato Yusuke, Mako Nakamura M, Yoshihide Ikeuchi Y (2013) Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLoS ONE 8:e80152
Inoue T, Hashimoto M, Katakura M, Tanabe Y, Al Mamun A, Matsuzaki K, Otani H, Shido O (2014) Effects of chronic administration of arachidonic acid on lipid profiles and morphology in the skeletal muscles of aged rats. Prostaglandins Leukot Essent Fatty Acids 91:119–127
Juman S, Hashimoto M, Katakura M, Inoue T, Tanabe Y, Arita M, Miki T, Shido O (2013) Effects of long-term oral administration of arachidonic acid and docosahexaenoic acid on the immune functions of young rats. Nutrients 5:1949–1961
Oe H, Hozumi T, Murata E, Matsuura H, Negishi K, Matsumura Y, Iwata S, Ogawa K, Sugioka K, Takemoto Y, Shimada K, Yoshiyama M, Ishikura Y, Kiso Y, Yoshikawa J (2008) Arachidonic acid and docosahexaenoic acid supplementation increases coronary flow velocity reserve in Japanese elderly individuals. Heart 94:316–321
Ishikura Y, Ikeda G, Akimoto K, Hata M, Kusumoto A, Kidokoro A, Kontani M, Kawashima H, Kiso Y, Koga Y (2009) Arachidonic acid supplementation decreases P300 latency and increases P300 amplitude of event-related potentials in healthy elderly men. Neuropsychobiology 60:73–79
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Hashimoto M, Shinozuka K, Gamoh S, Tanabe Y, Hossain MS, Kwon YM, Hata N, Misawa Y, Kunitomo M, Masumura S (1999) The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats. J Nutr 129:70–76
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Hashimoto M, Hossain MS, Shimada T, Yamasaki H, Fujii Y, Shido O (2001) Effects of docosahexaenoic acid on annular lipid fluidity of the rat bile canalicular plasma membrane. J Lipid Res 42:1160–1168
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Padykula HA, Gauthier GF (1963) Cytochemical studies of adenosine triphosphatase in skeletal muscle fibers. J Cell Biol 18:87
Gauthier Geraldine F et al (1966) Cytological studies of fiber types in skeletal muscle. J Cell Biol 28:333
Gamoh S, Hashimoto M, Hossain S, Masumura S (2001) Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol 28:266–270
Hossain MS, Hashimoto M, Gamoh S, Masumura S (1999) Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem 72:1133–1138
Gamoh S, Hashimoto M, Sugioka K, Hossain MS, Hata N, Misawa Y, Masumura S (1999) Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 93:237–241
Gorski J, Nawrocki A, Murthy M (1998) Characterization of free and glyceride-esterified long chain fatty acids in different skeletal muscle types of the rat. Mol Cell Biochem 178:113–118
Blackard WG, Li J, Clore JN, Rizzo WB (1997) Phospholipid fatty acid composition in type I and type II rat muscle. Lipids 32:193–198
Kriketos AD, Pan DA, Sutton JR, Hoh JF, Baur LA, Cooney GJ, Jenkins AB, Storlien LH (1995) Relationships between muscle membrane lipids, fiber type, and enzyme activities in sedentary and exercised rats. Am J Physiol 269:R1154–R1162
Nikolaidis MG, Petridou A, Mougios V (2006) Comparison of the phospholipid and triacylglycerol fatty acid profile of rat serum, skeletal muscle and heart. Physiol Res 55:259–265
Fiehn W, Peter JB, Mead JF, Gan-Elepano M (1971) Lipids and fatty acids of sarcolemma, sarcoplasmic reticulum, and mitochondria from rat skeletal muscle. J Biol Chem 246:5617–5620
Ayre KJ, Hulbert AJ (1996) Effects of changes in dietary fatty acids on isolated skeletal muscle functions in rats. J Appl Physiol 80:464–471
Infante RC, Kirwan JT (2001) Brenna, High levels of docosahexaenoic acid (22:6n-3)-containing phospholipids in high-frequency contraction muscles of hummingbirds and rattlesnakes. Compar Biochem Physiol Part B 130:291–298
Stark KD, Lim SY, Salem N Jr (2007) Docosahexaenoic acid and n-6 docosapentaenoic acid supplementation alter rat skeletal muscle fatty acid composition, 6:13S
Benzi G, Pastoris O, Marzatico F, Villa RF, Dagani F, Curti D (1992) The mitochondrial electron transfer alteration as a factor involved in the brain aging. Neurobiol Aging 13:361–368
Trounce I, Byrne E, Marzuki S (1989) Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet 1:637–639
Andrade FH, Reid MB, Allen DG, Westerblad H (1998) Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509:565–575
Andrade FH, Reid MB, Westerblad H (2001) Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15:309–311
Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F (1997) Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord 21:637–643. doi:10.1038/sj.ijo.0800451
Rivero JL, Talmadge RJ, Edgerton VR (1998) Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J Muscle Res Cell Motil 19:733–742
Rivero JL, Talmadge RJ, Edgerton VR (1999) Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem Cell Biol 111:277–287
Termin A, Staron RS, Pette D (1989) Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92:453–457
Jakobsson F, Borg K, Edstrom L (1990) Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol 80:459–468
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50:11–16
Welle S, Bhatt K, Thornton C (1996) Polyadenylated RNA, actin mRNA, and myosin heavy chain mRNA in young and old human skeletal muscle. Am J Physiol 270:E224–E229
Balagopal P, Schimke JC, Ades P, Adey D, Nair KS (2001) Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 280:E203–E208
Acknowledgments
This study was supported by a Health and Labour Sciences Research Grant of Japan (#H22-Shokuhin-Ippan-002) and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (#23500955, MH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare ‘no conflict of interest.’
Rights and permissions
About this article
Cite this article
Hashimoto, M., Inoue, T., Katakura, M. et al. Differential effects of docoosahexaenoic and arachidonic acid on fatty acid composition and myosin heavy chain-related genes of slow- and fast-twitch skeletal muscle tissues. Mol Cell Biochem 415, 169–181 (2016). https://doi.org/10.1007/s11010-016-2689-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2689-y