Abstract
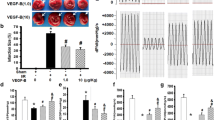
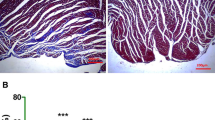
VEGF-C is a newly identified proangiogenic protein playing an important role in vascular disease and angiogenesis. However, its role in myocardial ischemia/reperfusion (I/R) injury remains unknown. The objective of this study was to determine the role and mechanism of VEGF-C in myocardial ischemia–reperfusion injury. Rat left ventricle myocardium was injected with recombinant human VEGF-C protein (0.1 or 1.0 µg/kg b.w.) 1 h prior to myocardial ischemia–reperfusion (I/R) injury. 24 h later, the myocardial infarction size, the number of TUNEL-positive cardiomyocytes, the levels of creatine kinase (CK), CK-MB, cardiac troponin, malondialdehyde (MDA) content, and apoptosis protein Bax expression were decreased, while Bcl2 and pAkt expression were increased in VEGF-C-treated myocardium as compared to the saline-treated I/R hearts. VEGF-C also improved the function of I/R-injured hearts. In the H2O2-induced H9c2 cardiomyocytes, which mimicked the I/R injury in vivo, VEGF-C pre-treatment decreased the LDH release and MDA content, blocked H2O2-induced apoptosis by inhibiting the pro-apoptotic protein Bax expression and its translocation to the mitochondrial membrane, and consequently attenuated H2O2-induced decrease of mitochondrial membrane potential and increase of cytochrome c release from mitochondria. Mechanistically, VEGF-C activated Akt signaling pathway via VEGF receptor 2, leading to a blockade of Bax expression and mitochondrial membrane translocation and thus protected cardiomyocyte from H2O2-induced activation of intrinsic apoptotic pathway. VEGF-C exerts its cardiac protection following I/R injury via its anti-apoptotic effect.









Similar content being viewed by others
References
Ibáñez B, Heusch G, Ovize M, Van de Werf F (2015) Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65:1454–1471
Reiter R, Swingen C, Moore L, Henry TD, Traverse JH (2012) Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res 110:105–110
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J Clin Investig 123:92–100
von Harsdorf R, Li PF, Dietz R (1999) Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99:2934–2941
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Tang JM, Wang JN, Zhang L (2011) VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res 91:402–411
Kajdaniuk D, Marek B, Foltyn W (2011) Vascular endothelial growth factor (VEGF): part 1: in physiology and pathophysiology. Endokrynol Pol 62:444–455
Dias S, Choy M, Alitalo K (2002) Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 99:2179–2184
Karpanen T, Heckman CA, Keskitalo S (2006) Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J 20:1462–1472
Muders MH, Zhang H, Wang E (2009) Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Res 69:6042–6048
Zhao T, Zhao W, Chen Y (2013) Differential expression of vascular endothelial growth factor isoforms and receptor subtypes in the infarcted heart. Int J Cardiol 167:2638–2645
Huang GQ, Wang JN, Tang JM, Zhang L, Zheng F, Yang JY, Guo LY, Kong X, Huang YZ, Liu Y, Chen SY (2011) The combined transduction of copper, zinc-superoxide dismutase and catalase mediated by cell-penetrating peptide, PEP-1, to protect myocardium from ischemia–reperfusion injury. J Transl Med 9:73
Mishra V, Baines M, Wenstone R (2005) Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem 42:269–276
Hou G, Xue L, Lu Z (2007) An activated mTOR/p70S6 K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett 253:236–248
Shin EJ, Schram K, Zheng XL (2009) Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol 221:490–497
Eguchi M, Liu Y, Shin EJ (2008) Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J 275:3136–3144
Cook SA, Sugden PH, Clerk A (1999) Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res 85:940–949
Valks DM, Kemp TJ, Clerk A (2003) Regulation of Bcl-xL expression by H2O2 in cardiac myocytes. J Biol Chem 278:25542–25547
Lee YS, Kang YJ, Kim HJ (2006) Higenamine reduces apoptotic cell death by induction of hemeoxygenase-1 in rat myocardial ischemia–reperfusion injury. Apoptosis 11:1091–1100
Zhang L, Dong XW, Wang JN (2012) PEP-1-CAT-transduced mesenchymal stem cells acquire an enhanced viability and promote ischemia-induced angiogenesis. PLoS One 7:e52537
Desbiens KM, Deschesnes RG, Labrie MM, Desfossés Y, Lambert H, Landry J, Bellmann K (2003) c-Myc potentiates the mitochondrial pathway of apoptosis by acting upstream of apoptosis signal-regulating kinase 1 (Ask1) in the p38 signalling cascade. Biochem J 372:631–641
Hou Q, Hsu YT (2005) Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am J Physiol Heart Circ Physiol 289:H477–H487
Sun B, Sun GB, Xiao J (2012) Isorhamnetin inhibits H (2) O (2)-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem 113:473–485
Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ (2013) Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J 34:1714–1722
Li R, Yan G, Li Q (2012) MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H2O2)-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One 7:e44907
Crow MT, Mani K, Nam YJ (2004) the mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 95:957–970
Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343
Fujio Y, Nguyen T, Wencker D (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia–reperfusion injury in mouse heart. Circulation 101:660–667
Fujio Y, Walsh K (1999) Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274:16349–16354
Jiang X, Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73:87–106
Zhao T, Zhao W, Meng W, Liu C, Chen Y, Gerling IC, Weber KT, Bhattacharya SK, Kumar R, Sun Y (2015) VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am J Transl Res 7:697–709
van Wamel AJ, Ruwhof C, van der Valk-Kokshoom LE, Schrier PI, van der Laarse A (2001) The role of angiotensin II, endothelin-1 and transforming growth factor-beta as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol Cell Biochem 218:113–124
Taimeh Z, Loughran J, Birks EJ, Bolli R (2013) Vascular endothelial growth factor in heart failure. Nat Rev Cardiol 10:519–530
Acknowledgments
This study was supported by grants from the National natural Science Foundation of China (81170095 to J.M.T, 81328002 to S.Y.C), Hubei Department Science (2014CFB644 to J.M.T), Hubei Education Department Science Foundation (T201112 to J.M.T), and the National Institutes of Health (HL123302, HL119053, and HL107526 to S.Y.C.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu-guang Chen and Yan-xia Lv are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Xg., Lv, Yx., Zhao, D. et al. Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol Cell Biochem 413, 9–23 (2016). https://doi.org/10.1007/s11010-015-2622-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2622-9