Abstract
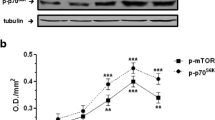
Cellular protein synthesis is believed to be antagonistically regulated by mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) signaling pathways. In the present study, we examined the relationship between mTOR/p70 S6 kinase (p70S6K) and AMPK in response to mechanical stretch. C2C12 myoblasts were grown on a silicone elastomer chamber to confluence and further cultured in differentiation medium for 4 days to form multinucleated myotubes. Cells were subjected to 15 % cyclic uniaxial stretch for 4 h at a frequency of 1 Hz. Phosphorylation of p70S6K at threonine 389 and AMPK at threonine 172 of the catalytic α subunit were concomitantly increased by mechanical stretch. Stimulation of the mTOR pathway by adding leucine and insulin increased the phosphorylation of p70S6K without inactivation of AMPK. In contrast, addition of compound C, a pharmacological inhibitor of AMPK, increased the phosphorylation of p70S6K in stretched cells. Activation of AMPK by the addition of 5-amino-4-imidazolecarboxamide ribonucleoside reduced the phosphorylation of p70S6K in response to mechanical stretch. In conclusion, crosstalk between mTOR and AMPK signaling was not tightly regulated in response to physiological stimuli, such as mechanical stress and/or nutrients. However, pharmacological modulation of AMPK influenced the mTOR/p70S6K signaling pathway.






Similar content being viewed by others
References
Lawrence JC Jr (2001) mTOR-dependent control of skeletal muscle protein synthesis. Int J Sport Nutr Exerc Metab 11(Suppl):S177–S185
Goodman CA, Mayhew DL, Hornberger TA (2011) Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23:1896–1906
Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10:457–468
Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB (2011) Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc 43:2249–2258
Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M (2007) S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 5:476–487
Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M (2005) Atrophy of S6K1(-/-) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7:286–294
Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95:1432–1437
Dennis PB, Pullen N, Kozma SC, Thomas G (1996) The principal rapamycin-sensitive p70(s6 k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol 16:6242–6251
Hornberger TA, Chien S (2006) Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem 97:1207–1216
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 103:4741–4746
Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA (2004) Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380:795–804
Nakai N, Kawano F, Oke Y, Nomura S, Ohira T, Fujita R, Ohira Y (2010) Mechanical stretch activates signaling events for protein translation initiation and elongation in C2C12 myoblasts. Mol Cells 30:513–518
Proud CG (2002) Regulation of mammalian translation factors by nutrients. Eur J Biochem 269:5338–5349
Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P (2006) Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr 136:264S–268S
Drummond MJ, Rasmussen BB (2008) Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 11:222–226
Stipanuk MH (2007) Leucine and protein synthesis: mTOR and beyond. Nutr Rev 65:122–129
Kimball SR, Jefferson LS (2006) New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 83:500S–507S
Nakai N, Shimomura Y, Tamura T, Tamura N, Hamada K, Kawano F, Ohira Y (2006) Leucine-induced activation of translational initiation is partly regulated by the branched-chain alpha-keto acid dehydrogenase complex in C2C12 cells. Biochem Biophys Res Commun 343:1244–1250
Du M, Shen QW, Zhu MJ, Ford SP (2007) Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci 85:919–927
Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC (2007) AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol 81:3649–3651
Kimball SR (2006) Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 38:1958–1964
Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Foretz M, Viollet B (2011) Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle 10:2640–2646
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887
Winder WW, Hardie DG (1996) Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 270:E299–E304
Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB (1997) Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem 272:13255–13261
Brozinick JT Jr, Birnbaum MJ (1998) Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem 273:14679–14682
Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS (1998) Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol 274:C406–C414
Bolster DR, Crozier SJ, Kimball SR, Jefferson LS (2002) AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277:23977–23980
Benziane B, Burton TJ, Scanlan B, Galuska D, Canny BJ, Chibalin AV, Zierath JR, Stepto NK (2008) Divergent cell signaling after short-term intensified endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 295:E1427–E1438
Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG (2010) Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42:1843–1852
Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM (2003) Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab 285:E854–E863
Wang L, Wang X, Proud CG (2000) Activation of mRNA translation in rat cardiac myocytes by insulin involves multiple rapamycin-sensitive steps. Am J Physiol Heart Circ Physiol 278:H1056–H1068
Gamble J, Lopaschuk GD (1997) Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 46:1270–1274
Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L (2001) Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett 505:348–352
Goodman CA (2014) The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol 166:43–95
Sasai N, Agata N, Inoue-Miyazu M, Kawakami K, Kobayashi K, Sokabe M, Hayakawa K (2010) Involvement of PI3 K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve 41:100–106
Thomson DM, Fick CA (1985) Gordon SE (2008) AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol 104:625–632
Jensen TE, Sylow L, Rose AJ, Madsen AB, Angin Y, Maarbjerg SJ, Richter EA (2014) Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca(2 +) release. Mol Metab 3:742–753
Miyamoto L, Egawa T, Oshima R, Kurogi E, Tomida Y, Tsuchiya K, Hayashi T (2013) AICAR stimulation metabolome widely mimics electrical contraction in isolated rat epitrochlearis muscle. Am J Physiol Cell Physiol 305:C1214–C1222
Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL (2012) Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS ONE 7:e52592
Ng TL, Leprivier G, Robertson MD, Chow C, Martin MJ, Laderoute KR, Davicioni E, Triche TJ, Sorensen PH (2012) The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell Death Differ 19:501–510
Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS (2005) Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol Endocrinol Metab 288:E980–E988
Valentine RJ, Coughlan KA, Ruderman NB, Saha AK (2014) Insulin inhibits AMPK activity and phosphorylates AMPK Ser(485/491) through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch Biochem Biophys 562:62–69
Schiaffino S, Mammucari C (2011) Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1:4
Barazzoni R, Short KR, Asmann Y, Coenen-Schimke JM, Robinson MM, Nair KS (2012) Insulin fails to enhance mTOR phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. Am J Physiol Endocrinol Metab 303:E1117–E1125
Padmasekar M, Sharifpanah F, Finkensieper A, Wartenberg M, Sauer H (2011) Stimulation of cardiomyogenesis of embryonic stem cells by nitric oxide downstream of AMP-activated protein kinase and mTOR signaling pathways. Stem Cells Dev 20:2163–2175
Funding
This work was supported by a Grant-in-Aid for Scientific Research C (25350813 to N.N.) from the Japan Society for the Promotion of Science (JSPS), Japan.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakai, N., Kawano, F. & Nakata, K. Mechanical stretch activates mammalian target of rapamycin and AMP-activated protein kinase pathways in skeletal muscle cells. Mol Cell Biochem 406, 285–292 (2015). https://doi.org/10.1007/s11010-015-2446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2446-7