Abstract
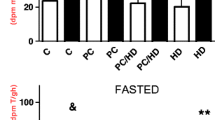
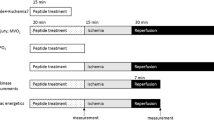
Ornithine decarboxylase (ODC) is the first rate-limiting enzyme in polyamine biosynthesis, which is essential for cell survival. We hypothesized that the ODC/polyamine system is involved in ischemic preconditioning (IPC)-mediated cardioprotection through the activation of Erk1/2 and Akt and through the inhibition of the mitochondrial permeability transition (mPT). Isolated rat hearts were subjected to 40 min of ischemia either with or without IPC (3 cycles of 5-min global ischemia), and ODC protein expression, polyamine content, and Akt and Erk1/2 phosphorylation were evaluated after 30 min of reperfusion. IPC significantly upregulated the ODC/polyamine pathway, promoted Erk1/2 and Akt phosphorylation, and reduced the infarct size and heart dysfunction after reperfusion. An inhibitor of ODC, α-difluoromethylornithine (DFMO), abolished the IPC-induced cardioprotection. Moreover, the inhibition of the IPC-induced activation of Erk1/2 and Akt using PD98059 or wortmannin downregulated the ODC/polyamine system. In separate studies, the Ca2+ load required to open the mPT pore was significantly lower in DFMO-treated cardiac mitochondria than in mitochondria from IPC hearts. Furthermore, spermine or spermidine significantly inhibited the mPT induced by CaCl2. These results suggest that IPC upregulates the ODC/polyamine system and mediates preconditioning cardioprotection, which may depend on the phosphorylation/activation of Erk1/2 and Akt and on the inhibition of the mPT during reperfusion.






Similar content being viewed by others
References
Hausenloy DJ, Yellon DM (2009) Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis 204(2):334–341
Fryer RM, Pratt PF, Hsu AK, Gross GJ (2001) Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296(2):642–649
Hausenloy DJ, Mocanu MM, Yellon DM (2004) Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res 63(2):305–312
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288(2):H971–H976
Uchiyama T, Engelman RM, Maulik N, Das DK (2004) Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 109(24):3042–3049
Halestrap AP, Clarke SJ, Khaliulin I (2007) The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 1767(8):1007–1031
Bhagatte Y, Lodwick D, Storey N (2012) Mitochondrial ROS production and subsequent ERK phosphorylation are necessary for temperature preconditioning of isolated ventricular myocytes. Cell Death Dis 3(7):e345
Weiss JN, Korge P, Honda HM, Ping P (2003) Role of the mitochondrial permeability transition in myocardial disease. Circ Res 93(4):292–301
Agostinelli E, Arancia G, Vedova LD, Belli F, Marra M, Salvi M, Toninello A (2004) The biological functions of polyamine oxidation products by amine oxidases: perspectives of clinical applications. Amino Acids 27(3–4):347–358
Toninello A, Salvi M, Mondovì B (2004) Interaction of biologically active amines with mitochondria and their role in the mitochondrial-mediated pathway of apoptosis. Curr Med Chem 11(17):2349–2374
Dypbukt JM, Ankarcrona M, Burkitt M, Sjöholm A, Ström K, Orrenius S, Nicotera P (1994) Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem 269(48):30553–30560
Turchanowa L, Rogozkin VA, Milovic V, Feldkoren BI, Caspary WF, Stein J (2000) Influence of physical exercise on polyamine synthesis in the rat skeletal muscle. Eur J Clin Invest 30(1):72–78
Desiderio MA, Tacchini L, Anzon E, Pogliaghi G, Radice L, Bernelli-Zazzera A (1996) Effects of polyamine imbalance on the induction of stress genes in hepatocarcinoma cells exposed to heat shock. Hepatology 24(1):150–156
Feith DJ, Bol DK, Carboni JM, Lynch MJ, Sass-Kuhn S, Shoop PL, Shantz LM (2005) Induction of ornithine decarboxylase activity is a necessary step for mitogen-activated protein kinase kinase-induced skin tumorigenesis. Cancer Res 65(2):572–578
Passariello CL, Gottardi D, Cetrullo S, Zini M, Stefanelli C et al (2012) Evidence that AMP-activated protein kinase can negatively modulate ornithine decarboxylase activity in cardiac myoblasts. Biochim Biophys Acta 1823(4):800–807
Hsu PC, Hung HC, Liao YF, Liu CC, Tsay GJ, Liu GY (2008) Ornithine decarboxylase attenuates leukemic chemotherapy drugs-induced cell apoptosis and arrest in human promyelocytic HL-60 cells. Leuk Res 32(10):1530–1540
Płoszaj T, Motyl T, Zimowska W, Skierski J, Zwierzchowski L (2000) Inhibition of ornithine decarboxylase by alpha-difluoromethylornithine induces apoptosis of HC11 mouse mammary epithelial cells. Amino Acids 19(2):483–496
Nitta T, Igarashi K, Yamamoto N (2002) Polyamine depletion induces apoptosis through mitochondria-mediated pathway. Exp Cell Res 276(1):120–128
Nitta T, Igarashi K, Yamamoto N (2012) Polyamine depletion enhances the roscovitine-induced apoptosis through the activation of mitochondria in HCT116 colon carcinoma cells. Amino Acids 42(2–3):655–665
Hegardt C, Andersson G, Oredsson SM (2003) Spermine prevents cytochrome c release in glucocorticoid-induced apoptosis in mouse thymocytes. Cell Biol Int 27(2):115–121
Zhao YJ, Xu CQ, Zhang WH, Zhang L, Bian SL, Huang Q, Sun HL, Li QF, Zhang YQ, Tian Y, Wang R, Yang BF, Li WM (2007) Role of polyamines in myocardial ischemia/reperfusion injury and their interactions with nitric oxide. Eur J Pharmacol 562(3):236–246
Zahedi K, Lentsch AB, Okaya T, Barone S, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Jänne J, Porter CW, Soleimani M (2009) Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 296(4):G899–G909
Rhee HJ, Kim EJ, Lee JK (2007) Physiological polyamines: simple primordial stress molecules. J Cell Mol Med 11(4):685–703
Zhao YJ, Zhang WH, Xu CQ, Li HZ, Wang LN, Li H, Sun YH, Lin Y, Han LP, Zhang L, Tian Y, Wang R, Yang BF, Li WM (2009) Involvement of the ornithine decarboxylase/polyamine system in precondition-induced cardioprotection through an interaction with PKC in rat hearts. Mol Cell Biochem 332(1–2):135–144
Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M (2005) Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 38(2):367–374
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42(1):39–51
Ray RM, Li C, Bhattacharya S, Naren AP, Johnson LR (2012) Spermine, a molecular switch regulating EGFR, integrin β3, Src, and FAK scaffolding. Cell Signal 24(4):931–942
Kurata HT, Marton LJ, Nichols CG (2006) The polyamine binding site in inward rectifier K+ channels. J Gen Physiol 127(5):467–480
Liu GY, Hung YC, Hsu PC, Liao YF, Chang WH, Tsay GJ, Hung HC (2005) Ornithine decarboxylase prevents tumor necrosis factor alpha-induced apoptosis by decreasing intracellular reactive oxygen species. Apoptosis 10(3):569–581
Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ (2001) Ornithine decarboxylase knockdown exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Cereb Blood Flow Metab 21(8):945–954
Woodcock EA, Lambert KA, Du XJ (1996) Ins (1,4,5) P3 during myocardial ischemia and its relationship to the development of arrhythmias. J Mol Cell Cardiol 28(10):2129–2138
Choi YH, Park HY (2012) Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci 19:31
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95(19):11140–11145
Chappell WH, Steelman LS, Long JM, Kempf RC, McCubrey JA et al (2011) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2(3):135–164
Strohm C, Barancik T, Brühl ML, Kilian SA, Schaper W (2000) Inhibition of the ER-kinase cascade by PD98059 and UO126 counteracts ischemic preconditioning in pig myocardium. J Cardiovasc Pharmacol 36(2):218–229
Mocanu MM, Bell RM, Yellon DM (2002) PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol 34(6):661–668
Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R (1999) PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol 276(5 Pt 2):H1468–H1481
Fryer RM, Pratt PF, Hsu AK, Gross GJ (2001) Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296(2):642–649
Shantz LM (2004) Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem J 377(Pt 1):257–264
Wei LH, Yang Y, Wu G, Ignarro LJ (2008) IL-4 and IL-13 upregulate ornithine decarboxylase expression by PI3K and MAP kinase pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol 294(5):C1198–C1205
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105(2):151–154
Halestrap AP (2010) A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38(4):841–860
Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP (2008) Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res 102(9):1082–1090
Lapidus RG, Sokolove PM (1992) Inhibition by spermine of the inner membrane permeability transition of isolated rat heart mitochondria. FEBS Lett 313(3):314–318
Lapidus RG, Sokolove PM (1993) Spermine inhibition of the permeability transition of isolated rat liver mitochondria: an investigation of mechanism. Arch Biochem Biophys 306(1):246–253
Lapidus RG, Sokolove PM (1994) The mitochondrial permeability transition. Interactions of spermine, ADP, and inorganic phosphate. J Biol Chem 269(29):18931–18936
Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A (2006) Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med 41(8):1272–1281
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81170178, 81170289, 81170218, 81100163, and 81100170).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Xue, G., Zhang, W. et al. Akt and Erk1/2 activate the ornithine decarboxylase/polyamine system in cardioprotective ischemic preconditioning in rats: the role of mitochondrial permeability transition pores. Mol Cell Biochem 390, 133–142 (2014). https://doi.org/10.1007/s11010-014-1964-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-1964-z