Abstract
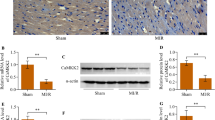
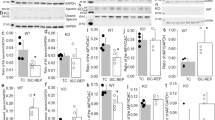
While Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been suggested to be an important protein regulating heart function upon ischemia/reperfusion (I/R), the mechanisms responsible are not fully known. Furthermore, it is not known whether CaMKII activation can modulate necroptosis, a recently described form of programmed cell death. In order to investigate these issues, Langendroff-perfused rat hearts were subjected to global ischemia and reperfusion, and CaMKII inhibition was achieved by adding the CaMKII inhibitor KN-93 (0.5 μmol/dm3) to the perfusion solution before the induction of ischemia. Immunoblotting was used to detect changes in expression of proteins modulating both necroptotic and apoptotic cell death. CaMKII inhibition normalized I/R induced increases in expression of necroptotic RIP1 and caspase-8 along with proteins of the intrinsic apoptotic pathway, namely cytochrome c and caspase-9. In addition, it increased the Bcl-2/Bax ratio and reduced caspase-3 and cleaved PARP1 content suggesting reduction of cell death. These changes coexisted with improvement of postischemic contractile function. On the other hand, there was no correlation between levels of pT287-CaMKIIδ and LVDP recovery after I/R. These results demonstrate for the first time that CaMKII inhibition may mitigate cardiac contractile dysfunction, at least partially, by limiting the contents of not only apoptotic, but also necroptotic proteins. Phosphorylation of CaMKII seems unlikely to determine the degree of postischemic recovery of contractile function.




Similar content being viewed by others
References
Hetz CA, Torres V, Quest AF (2005) Beyond apoptosis: nonapoptotic cell death in physiology and disease. Biochem Cell Biol 83(5):579–588
Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268
Moquin D, Chan FK (2010) The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35(8):434–441
Kung G, Konstantinidis K, Kitsis RN (2011) Programmed necrosis, not apoptosis, in the heart. Circ Res 108(8):1017–1036
Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP (2012) Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia–reperfusion in vivo. Basic Res Cardiol 107(4):270
Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM (2007) Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21(4):227–233
Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S (2012) Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81(8):751–761
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4(5):313–321
Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ (2011) Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab 31(1):178–189
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227
Opie LH, Thandroyen FT (1984) Molecular and biochemical mechanisms underlying the role of calcium ions in malignant ventricular arrhythmias. Ann N Y Acad Sci 427:127–139
Jennings RB, Steenbergen C, Reimer KA (1995) Myocardial ischemia and reperfusion. Monogr Pathol 37:47–80
Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM (2012) Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res 94(2):168–180
Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundiña-Weilenmann C, Vittone L, Mattiazzi A (2008) Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+ /calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol 295(4):H1669–H1683
Salas MA, Valverde CA, Sánchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A (2010) The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol 48(6):1298–1306
Wu Y, Shintani A, Grueter C, Zhang R, Hou Y, Yang J, Kranias EG, Colbran RJ, Anderson ME (2006) Suppression of dynamic Ca(2 +) transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J Mol Cell Cardiol 40(2):213–223
Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME (2005) Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11(4):409–417
Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A (2007) CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia–reperfusion injury. Cardiovasc Res 73(4):689–698
Adameova A, Carnicka S, Rajtik T, Szobi A, Nemcekova M, Svec P, Ravingerova T (2012) Upregulation of CaMKIIδ during ischemia–reperfusion is associated with reperfusion-induced arrhythmias and mechanical dysfunction of the rat heart: involvement of sarcolemmal Ca2 + -cycling proteins. Can J Physiol Pharmacol 90(8):1127–1134
Couchonnal LF, Anderson ME (2008) The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 23:151–159
Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H (1991) The newly synthesized selective Ca2 +/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12 h cells. Biochem Biophys Res Commun 181(3):968–975
Bell JR, Curl CL, Ip WT (2012) Delbridge LM (2012) Ca2 +/calmodulin-dependent protein kinase inhibition suppresses post-ischemic arrhythmogenesis and mediates sinus bradycardic recovery in reperfusion. Int J Cardiol 159(12):112–118
Braunwald E, Kloner RA (1985) Myocardial reperfusion: a double-edged sword? J Clin Invest 76(5):1713–1719
Corr PB, Witkowski FX (1984) Arrhythmias associated with reperfusion: basic insights and clinical relevance. J Cardiovasc Pharmacol 6(Suppl 6):S903–S909
Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol 82(3):249–258
Neuss M, Crow MT, Chesley A, Lakatta EG (2001) Apoptosis in cardiac disease–what is it–how does it occur. Cardiovasc Drugs Ther 15(6):507–523
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17(20):2481–2495
Chaitanya GV, Steven AJ, Babu PP (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31
Lundberg KC, Szweda LI (2004) Initiation of mitochondrial-mediated apoptosis during cardiac reperfusion. Arch Biochem Biophys 432(1):50–57
Chen X, Zhang X, Kubo H et al (2005) Ca2 + influx-induced sarcoplasmic reticulum Ca2 + overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res 97(10):1009–1017
Szabadkai G, Rizzuto R (2004) Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett 567(1):111–115
Bagnoli M, Canevari S, Mezzanzanica D (2010) Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol 42(2):210–213
Yang BF, Xiao C, Li H, Yang SJ (2007) Resistance to Fas-mediated apoptosis in malignant tumours is rescued by KN-93 and cisplatin via downregulation of c-FLIP expression and phosphorylation. Clin Exp Pharmacol Physiol 34(12):1245–1251
Xiao C, Yang BF, Song JH, Schulman H, Li L, Hao C (2005) Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp Cell Res 304(1):244–255
Acknowledgments
Authors would like to thank Mrs. V. Hassova for her indispensable help. This study was supported by Grants UK/430/2013, VEGA 1/0638/12, VEGA 2/0054/11, APVV 0102-11, and APVV 0523-10.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szobi, A., Rajtik, T., Carnicka, S. et al. Mitigation of postischemic cardiac contractile dysfunction by CaMKII inhibition: effects on programmed necrotic and apoptotic cell death. Mol Cell Biochem 388, 269–276 (2014). https://doi.org/10.1007/s11010-013-1918-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1918-x