Abstract
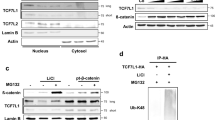
Tcf3 acts as a transcription factor controlling gene expression in canonical Wnt signaling. In this study we show that mouse Tcf3 represses canonical Wnt signaling in mouse neural stem cells and in human HEK 293 cells. We demonstrate that mouse Tcf3 mediates repression of both moderate and high levels of canonical Wnt signaling, by either competing with other members of the Tcf/Lef family for binding to β-catenin, or for binding to DNA. We observed that the repressor activity of mouse Tcf3 was only relieved effectively upon simultaneous disruption of both mechanisms. Immunofluorescence of transfected HEK 293 cells showed co-localization of β-catenin and Tcf3 in the nucleus of cells transfected with full-length Tcf3, but not in cells transfected with N-terminal deleted versions. A direct physical interaction between β-catenin and Tcf3 in the nucleus was confirmed by co-immunoprecipitation studies. The inhibitory β-catenin/Tcf3 interface was independent of the ability of Tcf3 to directly interact with DNA.




Similar content being viewed by others
References
Hoppler S, Kavanagh CL (2007) Wnt signalling: variety at the core. J Cell Sci 120:385–393
Arce L, Yokoyama NN, Waterman ML (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25:7492–7504
Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ (2011) Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13:762–770
Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA (2008) Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22:746–755
Pereira L, Yi F, Merrill BJ (2006) Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol 26:7479–7491
Yi F, Pereira L, Merrill BJ (2008) Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells 26:1951–1960
Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B (2008) T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells 26:2019–2031
Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J (2002) Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129:4015–4025
Gradl D, Konig A, Wedlich D (2002) Functional diversity of Xenopus lymphoid enhancer factor/T-cell factor transcription factors relies on combinations of activating and repressing elements. J Biol Chem 277:14159–14171
Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB (2000) Repressor activity of headless/Tcf3 is essential for vertebrate head formation. Nature 407:913–916
Merrill BJ, Gat U, DasGupta R, Fuchs E (2001) Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 15:1688–1705
Nguyen H, Rendl M, Fuchs E (2006) Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127:171–183
Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E (2009) Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet 41:1068–1075
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40:499–507
van den Bout CJ, Machon O, Rosok O, Backman M, Krauss S (2002) The mouse enhancer element D6 directs Cre recombinase activity in the neocortex and the hippocampus. Mech Dev 110:179–182
Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100:3299–3304
Machon O, van den Bout CJ, Backman M, Rosok O, Caubit X, Fromm SH, Geronimo B, Krauss S (2002) Forebrain-specific promoter/enhancer D6 derived from the mouse Dach1 gene controls expression in neural stem cells. Neuroscience 112:951–966
von Kries JP, Winbeck G, Asbrand C, Schwarz-Romond T, Sochnikova N, Dell’Oro A, Behrens J, Birchmeier W (2000) Hot spots in beta-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol 7:800–807
Graham TA, Weaver C, Mao F, Kimelman D, Xu W (2000) Crystal structure of a beta-catenin/Tcf complex. Cell 103:885–896
Love JJ, Li X, Case DA, Giese K, Grosschedl R, Wright PE (1995) Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376:791–795
Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S (2007) A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol 311:223–237
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R (1996) Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 59:3–10
Krieghoff E, Behrens J, Mayr B (2006) Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J Cell Sci 119:1453–1463
Jamieson C, Sharma M, Henderson BR (2011) Regulation of β-catenin nuclear dynamics by GSK-3beta involves a LEF-1 positive feedback loop. Traffic 12:983–999
Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia–Garcia MJ, Anderson KV, Fuchs E (2004) Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131:263–274
Miravet S, Piedra J, Miro F, Itarte E, de Garcia HA, Dunach M (2002) The transcriptional factor Tcf-4 contains different binding sites for beta-catenin and plakoglobin. J Biol Chem 277:1884–1891
Moon RT, Kimelman D (1998) From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays 20:536–545
Solberg N, Machon O, Krauss S (2008) Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev Dyn 237:1799–1811
Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S (2003) Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience 122:129–143
Lee SM, Tole S, Grove E, McMahon AP (2000) A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127:457–467
Acknowledgment
This research was supported by a grant of the Research Council of Norway. Grant no 174938.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solberg, N., Machon, O., Machonova, O. et al. Mouse Tcf3 represses canonical Wnt signaling by either competing for β-catenin binding or through occupation of DNA-binding sites. Mol Cell Biochem 365, 53–63 (2012). https://doi.org/10.1007/s11010-012-1243-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1243-9