Abstract
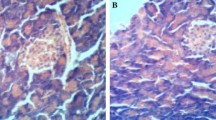
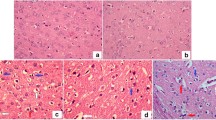
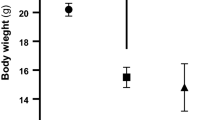
Diabetes mellitus manifests itself in a wide variety of complications and the symptoms of the disease are multifactorial. The present study was carried out to investigate the effects of vanadyl sulfate on biochemical parameters, enzyme activities and brain lipid peroxidation, glutathione and nonenzymatic glycosylation of normal- and streptozotocin-diabetic rats. Streptozotocin (STZ) was administered as a single dose (65 mg/kg) to induce diabetes. A dose of 100 mg/kg vanadyl sulfate was orally administered daily to STZ-diabetic and normal rats, separately until the end of the experiment, at day 60. In STZ-diabetic group, blood glucose, serum sialic and uric acid levels, serum catalase (CAT) and lactate dehydrogenase (LDH) activities, brain lipid peroxidation (LPO) and nonenzymatic glycosylation (NEG) increased, while brain glutathione (GSH) level and body weight decreased. In the diabetic group given vanadyl sulfate, blood glucose, serum sialic and uric acid levels, serum CAT and LDH activities and brain LPO and NEG levels decreased, but brain GSH and body weight increased.The present study showed that vanadyl sulfate exerted antioxidant effects and consequently may prevent brain damage caused by streptozotocin-induced diabetes.
Similar content being viewed by others
References
Jung CH, Seog HM, Choi IW, Choi HD, Cho HY: Effects of wild ginseng (Panaxginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzymeactivities in streptozotocin diabetic rats. J Ethnopharmacol 98: 245–250, 2005
Baynes JW: Role of oxidative stress in development of complications indiabetes. Diabetes 40: 405–412, 1991
Ceriello A: Oxidative stress and glycemic regulation. Metabolism 49:27–29, 2000
Young IS, Tate S, Lightbody JH, Mc Master D, Trimble ER: The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocindiabetic rat. Free Radic Biol Med 18: 833–840, 1995
Baynes JW,Thorpe SR: Role of oxidative stress in diabetic complications:a new perspective on an old paradigm. Diabetes 48: 1–9,1999
Bhor VM, Raghuram N, Sivakami S: Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabeticrats. Int Biochem Cell Biol 36: 89–97, 2004
Cusi K, Cukier S, Defronzo R, Torres M, Puchulu FM, Redondo JCP:Vanadyl sulfate improves hepatic and muscle insulin sensitivity in type 2 diabetes.J Clin Endocrinol Metab 86: 1410–1417, 2001
Yanardag R, Bolkent S, Karabulut-Bulan O, Tunali S: Effects of vanadyl sulfate on kidney in experimental diabetes. Biol Trace Elem Res 95:73–85, 2003
Jound A, Lambert AE, Stauffacher W, Renold AE: Diabetogenic action of streptozotocin. Relationship of dose to metabolic response. J Clin Invest48: 2129–2139, 1969
Relander A, Raiha CE: Differences between the enzymatic and toluidinemethods of blood glucose determination. Scand J Clin Lab Invest 15: 221–224, 1963
Lorentz K, Weiβ T, Kraas E: Sialic acid in human serum andcerebrospinal fluid. J Clin Chem Clin Biochem 24: 189–198, 1986
Caraway WT: Determination of uric acid in serum by a carbonate method. AmJ Clin Pathol 25: 840–845, 1955
Aebi H: Catalase in vitro. Methods Enzymol 105: 121–126, 1984
Wroblewski F: Clinical significance of serum enzyme alterationsassociated with mycardial infarction. Am Heart J 54: 219–224, 1957
Beutler E: Glutathione in red cell metabolism: a manuel of biochemicalmethods. 2nd ed, Grune and Stratton, New York, 1975, p 112–114
Ledwozyw A, Michalak J, Stepien A, Kadziolka A: The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clin Chim Acta 155: 275–283,1986
Parker KM, England JD, Casto JD, Hessel R, Goldstein PE: Improved colorimetric assay for glycosylated hemoglobin. Clin Chem 27:669–672, 1981
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement withthe folin phenol reagent. J Biol Chem 193: 265–275, 1951
Musabayane CT, Mahlalela N, Shode FO, Ojewole JAO: Effects of Syzygium cordatum (Hochst.) [Myrtaceae] leaf extract on plasma glucose and hepatic glycogen instreptozotocin-induced rats. J Ethnopharmacol 97: 485–490, 2005
David MN, James M, Daniel ES: The epidemiology of cardiovascular disease type 2 diabetes mellitus: how sweet it is⋖ or is it? Lancet 350:S14–S19, 1997
Luzi L: Pancreas transplantation and diabetic complications. N Engl JMed 339: 115–117, 1998
Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ: Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol CellBiochem 224: 45–51, 2001
Rajkumar L, Srinivasan N, Balasubramanian K, Govindarajulu P: Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol 29:1081–1083, 1991
Ravi K, Ramachandran B, Subramanian S: Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin-induced diabetic rats. Biol PharmBull 27: 1212–1217, 2004
Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler H, Nawroth PP: The role of oxidative stress and NF (B) activation in late diabeticcomplications. Biofactors 10: 175–179, 1999
Donnini D, Zambito AM, Perella G, Ambesi-Impiombat O, Curcio F: Glucose may induce cell death through a free radical-mediated mechanism. BiochemBiophys Res Commun 219: 412–417, 1996
Arun N, Nalini N: Efficacy of turmeric on blood sugar and polyol pathwayin diabetic albino rats. Plant Food Hum Nutr 57: 41–52, 2002
Bolkent S, Bolkent S, Yanardag R, Tunali S: Protective effect of vanadyl sulfate on the pancreas of streptozotocin-induced diabetic rats. DiabetesRes Clin Pract 70: 103–109, 2005
Mohamed AAM, Mayra A, Thanaa MKR, Nabila Al-S, Nashami AS, Joseph EG: Association of serum sialic acid with cardiovascular metabolic risk factors in Kuwaiti children and adolescents with type 1 diabetes. Metabolism 53:638–643, 2004
Gavella M, Lipovac V, Car A, Vučić M, Sokolić L, Rakoš R: Serum sialic acid in subjects with impaired glucose tolerance and in newlydiagnosed type 2 diabetic patients. Acta Diabetol 40: 95–100, 2003
Crook MA, Tutt P, Pickup JC: Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care16: 57–60, 1993
Sillanaukee P, Ponnio M, Jaaskelainen IP: Occurrence of sialic acid inhealthy humans and different disorders. Eur J Clin Invest 29: 413–425, 1999
Sonmez H, Suer S, Gungor Z, Kokoglu E, Ispir T: Sialidase activities and sialic acid levels of the liver and serum from streptozotocin induceddiabetic rats. Diabetes Res 32: 101–106, 1997
Sung KC, Kim BS, Kang JH, Lee MH, Park JR,Rhee EJ, Lee WY, Kim SW, Kim H, Lee KB,Ryu SH: In normoglycemic Koreans, insulin resistance and adipocity are independently correlated with high blood pressure. Circ J 68 : 898–902,2004
Yoo TW, Sung KC, Shin HS, Kim BJ, Kim BS, Kang JH et al. Relationship between serum uric acid concentration and insulin resistance and metabolicsyndrome. Circ J 69: 928–933, 2005
Zavaroni I, Mazza S, Fantuzzi M, Dall'Aglio E, Bonora E, Delsignore R et al. Changes in insulin and lipid metabolism in males with asymptomatichyperuricaemia. J Intern Med 234: 25–30, 1993
Cigolini M, Targher G, Tonoli M, Manara F, Muggeo M, De Sandre G: Hyperuricaemia: relationships to body fat distribution and other components of the insulin resistance sydrome in 38 years old healthy men and women. IntJ Obes Relat Metab Disord 19: 92–96, 1995
Yanardag R, Ozsoy-Sacan O, Orak H, Ozgey Y: Protective effects of glurenorm (qliquidone) treatment on the liver injury of experimentaldiabetes. Drug Chem Toxicol 28: 483–497, 2005
Rajasekaran S, Sivagnanam K, Subramanian S: Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol. Reports 57:90–96, 2005
Gonzales R, Auclair C, Voisin E, Gantero H, Dhermy D, Boivin P: Superoxide dismutase, catalase, and glutathione peroxidase in red blood cells from patients with malignant diseases. Cancer Res 4:4137–4139, 1984
Kakkar R, Kalra J, Mantha SV, Prasad K: Lipid peroxidation and activityof antioxidant enzymes in diabetic rats. Mol Cell Biochem 151: 113–119, 1995
Aldrich JE: Clinical enzymology. In clinical chemistry concepts and applications (eds.) Anderson, S.C.; Cockayne, S. New York: McGraw Hill;2003, 261–284
Navarro CM, Montilla, PM, Martin A, Jimenez J, Utrilla PM: Free radicalsscavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med 59: 312–314, 1993
Ozkaya YG, Agar A, Yargicoglu P, Hacioglu G, Bilmen-Sarikcioglu S, Ozen I, Aliciguzel Y: The effect of exercise on brain antioxidant status of diabetic rats. Diabetes Metab 28: 377–384, 2002
Kumar JSS, Menon VP: Effect of diabetes on levels of lipid peroxides and glycolipids inrat brain. Metabolism 42: 1435–1439, 1993
Nishikawa T, Edelstein D, Du XL: Normalizing mitochondrial superoxide produciton blocks three pathways of hyperglycaemic damage. Nature 404:787–790, 2000
Chiueh CC: Neuroprotective properties of nitric oxide. Ann. NY Acad Sci 890: 301–311,1999
Bromme HJ, Morke W, Peschke D, Ebelt H, Peschke D: Scavenging effect of melatonin on hydroxyl radicals generated by alloxan. J Pineal Res 29:201–208, 2000
Esterbauer H, Zollner H, Schaur RJ: Lipid Oxidation. C. Vigo and BA Pel Frey (eds). CRC Press: Boca Raton, FL 1990
Travero N, Menini S, Cosso L, Odetti E, Albano E, Pronzato MA, Marinari Um: Immunological evidence for increased oxidative stress in diabetic rats.Diabetologia 41: 265–270, 1998
Aragno M, Brignardello E, Tamagno DO, Boccuzzi G: Dehydroepiandrosterone administration prevents the oxidative damage induced by acute hyperglycemiain rats. J Endocrinol 155: 233–240, 1997
Suzuki WA, Clayton NS: The hippocampus and memory: a comparative andethnological perspective. Curr Opin Neurobiol 10: 768–773, 2000
Aksenov MY, Markesbery WR: Changes in thiol content and expression of glutamate redox system genes in the hippocampus and cerebellum inAlzheimer's disease. Neuroscience 302: 141–145, 2001
Conklin KA: Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effetcs. Nutr. Cancer37: 1–18, 2000
Rotruck JT, Pope AL, Ganther HE, Swanson AB: Selenium: Biochemicalroles as a component of glutathione peroxidase. Science 179: 588–590, 1973
Matcovis B, Varga SI, Szaluo L, Witsas H: The effect of diabetes on the activities of the peroxide metabolic enymes. Horm Metab Res 14:77–79, 1982
Yu BP: Cellular defense against damage from reactive oxygen species.Physiol Rev 1994, 74: 139–162, 1994
Asgary S, Naderi G, Sarrafzadegan N, Ghassemi N, Boshtam M, Rafie M, Arefian A: Anti-oxidant effect of flavonoids on hemoglobinglycosylation. Pharm Acta Helv 73: 223–226, 1999
Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A: Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr 20: 212–218,2001
Lapenna D, Ciofani G, Bruno C, Pirdomenico SD, Giuliani L, Giamberardino MA, Cuccurullo F: Vanadyl as a catalyst of human lipoprotein oxidation.Biochem Pharmacol 63: 375–380, 2002
Badmaev V, Prakash S, Majeed M: Vanadium: A review of its potential rolein the fight against diabetes. J Altern Complement Med 5: 273–291, 1999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yanardag, R., Tunali, S. Vanadyl Sulfate Administration Protects the Streptozotocin-Induced Oxidative Damage to Brain Tissue in Rats. Mol Cell Biochem 286, 153–159 (2006). https://doi.org/10.1007/s11010-005-9107-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-9107-1