Abstract
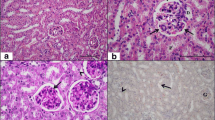
Melatonin and caffeic acid phenethyl ester (CAPE), a component of honeybee propolis, were recently found to be potent free radical scavengers and antioxidants. There are a number of reports on the effects induced by electromagnetic radiation (EMR) in various cellular systems. Mechanisms of adverse effects of EMR indicate that reactive oxygen species may play a role in the biological effects of this radiation. The present study was carried out to compare the protective effects of melatonin and CAPE against 900 MHz EMR emitted mobile phone-induced renal tubular injury. Melatonin was administered whereas CAPE was given for 10 days before the exposure. Urinary N-acetyl-β-D-glucosaminidase (NAG, a marker of renal tubular injury) and malondialdehyde (MDA, an index of lipid peroxidation), were used as markers of oxidative stress-induced renal impairment in rats exposed to EMR. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activities were studied to evaluate the changes of antioxidant status in renal tissue. Urinary NAG and renal MDA were increased in EMR exposed rats while both melatonin and CAPE caused a significant reduction in the levels of these parameters. Likewise, renal SOD and GSH-Px activities were decreased in EMR exposed animals while melatonin caused a significant increase in the activities of these antioxidant enzymes but CAPE did not. Melatonin caused a significant decrease in urinary NAG activity and MDA levels which were increased because of EMR exposure. CAPE also reduced elevated MDA levels in EMR exposed renal tissue, but the effect of melatonin was more potent than that of CAPE. Furthermore, treatment of EMR exposed rats with melatonin increased activities of SOD and GSH-Px to higher levels than those of control rats. In conclusion, melatonin and CAPE prevent renal tubular injury by reducing oxidative stress and protect the kidney from oxidative damage induced by 900 MHz mobile phone. Nevertheless, melatonin seems to be a more potent antioxidant compared with CAPE in kidney. (Mol Cell Biochem 276: 31–37, 2005)
Similar content being viewed by others
References
Cox DR: Communication of risk: health hazards from mobile phones. J Royal Statis Soc: Series A (Statis Soc) 166: 241–245, 2003
Knave B: Electromagnetic fields and health outcomes. Ann Acad Med 30(5): 489–493, 2001
Repacholi MH: Health risks from the use of mobile phones. Toxicol Lett 120: 323–331, 2001
Irmak MK, Fadillioglu E, Gulec M, Erdogan H, Yagmurca M, Akyol O: Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem Funct 20: 279–283, 2002
Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M, Akyol O, Ozen S: Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Acta 340: 153–162, 2004
Irmak MK, Oztas E, Yagmurca M, Fadillioglu E, Bakir B: Effects of electromagnetic radiation from a cellular telephone on epidermal Merkel cells. J Cutan Pathol 30: 135–138, 2003
Serel TA, Ozguner F, Soyupek S: Prevention of shock wave-induced renal oxidative stress by melatonin: An experimental study. Urol Res 32: 69–71, 2004
Raab WP: Diagnostic value of urinary enzyme determinations. Clin Chem 18: 5–25, 1972
Bosomworth MP, Aparicio SR, Hay AWM: Urine N-acetyl-β-D-glucosaminidase – A marker of tubular damage. Nephrol Dial Transplant 14: 620–666, 1999
Reiter RJ, Tan DX, Osuna C: Actions of melatonin in the reduction of oxidative stress. J Biomed Sci 7: 444–458, 2000
Reiter RJ, Tan DX, Cabrera J: The oxidant/antioxidant network: role of melatonin. Biol Signals Recep 8: 56–63, 1999
Baydas G, Ercel E, Canatan H: Effect of melatonin on oxidative status of rat brain, liver and kidney tissues under constant light exposure. Cell Biochem Funct 19: 37–41, 2001
Rao C, Desai D, Kaul B, Amin S, Reddy BS: Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chem Biol Interact 84: 277–290, 1992
Sud’ina GF, Mirzoeva OK, Pushkareva GA, Korshunova GA, Sumbatyan NV, Varfolomeev SD: Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett 329: 21–24, 1993
Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ: Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in rat model of inflammation. Cancer Res 59: 2347–2352, 1999
Chen YJ, Shiao MS, Wang SY: The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs 12: 143–149, 2001
Fesen MR, Pommier Y, Leteurtre E, Hiroguchi S, Yung J, Kohn KW: Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol 48: 595–608, 1994
Park EH, Kahng JH: Suppressive effects of propolis in rat adjuvant arthritis. Arch Pharm Res 22: 554–558, 1999
Ozguner F, Armagan A, Koyu A, Calıskan S, Koylu H: A novel antioxidant agent caffeic acid phenethyl ester (CAPE) prevents shock wave-induced renal tubular oxidative stress. Urol Res (in press), 2005
Gurel A, Armutcu F, Sahin S, Sogut S, Ozyurt H, Gulec M, Kutlu NO, Akyol O: Protective role of alphatocopherol and caffeic acid phenethyl ester on ischemia reperfusion injury via nitric oxide and myeloperoxidase in rat kidneys. Clin Chim Acta 339: 33–41, 2004
Uz E, Sogut S, Sahin S, Var A, Ozyurt H, Gulec M, Akyol O: The protective role of caffeic acid phenethyl ester (CAPE) on testicular tissue after testicular torsion and detorsion. World J Urol 20: 264–270, 2002
Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N: Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol 18: 234–238, 2004
Draper HH, Hadley M: Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186: 421–431, 1990
Yakata M, Sugita O, Sakai T, Uchiyama K, Wada K: Urinary enzyme determination and its clinical significance. C. Enzyme derived from the kidney epithelium-N acetyl.beta-D-glucosaminidase. 4. Preclinical evaluation of the urinary NAG activity and changes in the renal disease. Rinsho Byori 56: 90–101, 1983
Sun Y, Oberley LW, Li Y: A simple method for clinical assay of superoxide dismutase. Clin Chem 34: 497–500, 1988
Durak I, Yurtarslani Z, Canbolat O, Akyol O: A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin Chim Acta 214: 103–104, 1993
Paglia DE, Valentine WN: Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169, 1967
Aebi Y: Catalase in vitro. Methods enzymol 105: 121–126, 1984
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Clin Chem 193: 265–269, 1951
Vural H, Sabuncu T, Arslan SO, Aksoy N: Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J Pineal Res 31: 193–198, 2001
Bortkiewicz A: A study on the biological effects of exposure mobile-phone frequency EMF. Med Pr 52(2): 101–106, 2001
Riu PJ, Foster KR, Blick DW, Adair ER: A thermal model for human thresholds of microwave-evoked warmth sensations. Bioelectromagnetics 18(8): 578–583, 1997
Siu AW, Reiter RJ, To CH: The efficacy of vitamin E and melatonin as an oxidant against lipid peroxidation in rat retinal homogenates. J Pineal Res 24: 229–244, 1998
Siu AW, Reiter RJ, To CH: Pineal indolamines and vitamin E reduce nitric oxide-induced lipid peroxidation in rat retinal homogenates. J Pineal Res 27: 122–128, 1999
Irmak MK, Koltuksuz U, Gulec M, Kutlu NO, Yagmurca M, Ozyurt H, Karaman A, Akyol O: The effect of caffeic acid phenethyl ester ischemia reperfusion-induced injury in comparison with κ-tocopherol in rat kidney. Urol Res 29: 190–193, 2001
Baydas G, Canatan H, Turkoglu A: Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J Pineal Res 32: 225–228, 2002
Liu F, HG TB: Effect of pineal indoles of the antioxidant enzymes superoxide dismutase, catalase glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol 78: 447–453, 2000
Tan DX, Poeggeler B, Reiter RJ, Chen LD, Chen S: Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocrine J 1: 57–60, 1993
Tan DX, Manchester LJ, Reiter R: Melatonin directly scavenges hydrogen peroxide: a potentially new pathway of melatonin biotransformation. Free Radic Biol Med 29: 1177–1185, 2000
Antolin I, Rodrigez C, Sainz RM: Neurohormone melatonin prevents cell damage. Effect on gene expression for antioxidant enzymes. FASEB J 10: 882–890, 1996
Okatani Y, Wakatsiku A, Shinohara K, Kaneda C, Fukaya T: Melatonin stimulates Glutathione peroxidase activity in human chorion. J Pineal Res 30: 199–205, 2001
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozguner, F., Oktem, F., Armagan, A. et al. Comparative analysis of the protective effects of melatonin and caffeic acid phenethyl ester (CAPE) on mobile phone-induced renal impairment in rat. Mol Cell Biochem 276, 31–37 (2005). https://doi.org/10.1007/s11010-005-2734-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-2734-8