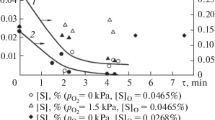
We consider the conditions of heat and mass exchange for drops of sulfuric acid in a gas flow and develop a mathematical model that enables us to determine the main parameters of evaporation of the dispersed solutions of sulfuric acid under the conditions of their direct contact with hot gases.
Similar content being viewed by others
References
V. T. Yavors’kyi, Technology of Sulfur and Sulfuric Acid [in Ukrainian], “L’vivs’ka Politekhnika” University, Lviv (2010).
K. M. Malin, Handbook for Workers in the Production of Sulfuric Acid [in Russian], Khimiya, Moscow (1971).
Ya. A. Kalymon, A. B. Helesh, and O. Ye. Yavorskyi, “Study of the process of boiling down of sulfuric acid solutions in a hollow dispersing column,” Ènergotekhnol. Resursosber., No. 4, 64–71 (2014).
Ya. A. Kalymon, A. B. Helesh, and O. Ye. Yavorskyi, “Hydrolytic sulfate acid evaporation by waste gases from burning furnaces of meta-titanic acid paste,” Chem. Chem. Technol., No. 6 (4), 423–429 (2012).
N. A. Fuks, Evaporation and Growth of Drops in Gaseous Atmospheres [in Russian], Izd. AN SSSR, Moscow (1958).
A. A. Lushnikov, V. A. Zagainov, and V. M. Nuzhnyi, “Evaluation of the rate of evaporation of the drops of water and the comparison with the experimental data,” Fiz. Aerodispers. Sist., No. 38, 7–17 (2001).
A. V. Kozyrev and A. G. Sitnikov, “Evaporation of a spherical drop in a medium-pressure gas,” Uspekhi Fiz. Nauk, 171, No. 7, 765–774 (2001).
A. L. Emel’yanov and E. S. Platunov, “Kinetics of evaporation of drops in the cooling systems of thermally loaded elements of devices,” Izv. Vyssh. Uchebn. Zaved. Pribor., 54, No. 1, 84–88 (2011).
M. O. Dikii, A. S. Solomakha, and V. G. Petrenko, “Mathematical simulation of the evaporation of water drops in air flows,” Vost.-Evrop. Zh. Peredov. Tekhnol., 3, No. 10 (63), 17–20 (2013).
S. E. Selivanov and M. I. Kulik, “Kinetics of evaporation of the drops of liquid fuels,” Vest. KhNADU, No. 52, 105–109 (2011).
R. S. Volkov, G. V. Kuznetsov, and P. A. Strizhak, “Effect of solid inclusions in liquid drops on the characteristics of their evaporation in motion through a high-temperature gaseous atmosphere,” Zh. Tekh. Fiz., 84, No. 12, 33–37 (2014).
A. G. Kasatkin, Basic Processes and Installations in Chemical Technologies [in Russian], Goskhimizdat, Moscow (1961).
V. S. Shvydkii and M. G. Ladygichev, Purification of Gases. A Handbook [in Russian], Teploénergetik, Moscow (2002).
D. G. Pazhi and V. S. Galustov, Foundations of the Technique of Spraying of Liquids [in Russian], Khimiya, Moscow (1984).
Ya. M. Khanyk, A. I. Dubynin, V. M. Atamanyuk, and O. V. Stanislavchuk, Processes and Devices in Chemical Technologies [in Ukrainian], “L’vivs’ka Politekhnika” University, Lviv (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 51, No. 5, pp. 90–97, September–October, 2015. Original article submitted July 1, 2015.
Rights and permissions
About this article
Cite this article
Yavors’kyi, V.T., Helesh, A.B. Determination of the Parameters of Evaporation of the Solutions of Sulfuric Acid with low Corrosion Activity of the Phases. Mater Sci 51, 691–700 (2016). https://doi.org/10.1007/s11003-016-9892-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-016-9892-6