Abstract
Objectives
To examine the relationships between gestational diabetes mellitus (GDM) treatment and neonatal anthropometry.
Methods
Covariate-adjusted multivariable linear regression analyses were used in 9907 offspring of the Born in Bradford cohort. GDM treatment type (lifestyle changes advice only, lifestyle changes and insulin or lifestyle changes and metformin) was the exposure, offspring born to mothers without GDM the control, and birth weight, head, mid-arm and abdominal circumference, and subscapular and triceps skinfold thickness the outcomes.
Results
Lower birth weight in offspring exposed to insulin (− 117.2 g (95% CI − 173.8, − 60.7)) and metformin (− 200.3 g (− 328.5, − 72.1)) compared to offspring not exposed to GDM was partly attributed to lower gestational age at birth and greater proportion of Pakistani mothers in the treatment groups. Higher subscapular skinfolds in offspring exposed to treatment compared to those not exposed to GDM was partly attributed to higher maternal glucose concentrations at diagnosis. In fully adjusted analyses, offspring exposed to GDM treatment had lower weight, smaller abdominal circumference and skinfolds at birth than those not exposed to GDM. Metformin exposure was associated with smaller offspring mid-arm circumference (− 0.3 cm (− 0.6, − 0.07)) than insulin exposure in fully adjusted models with no other differences found.
Conclusions for Practice
Offspring exposed to GDM treatment were lighter and smaller at birth than those not exposed to GDM. Metformin-exposed offspring had largely comparable birth anthropometric characteristics to those exposed to insulin.
Significance
What is already known on this subject? Offspring exposed to untreated GDM are more likely to have higher weight and adiposity at birth in comparison to offspring not exposed to GDM.
What this study adds? Offspring exposed to GDM treatment of any type (lifestyle changes advice, insulin or metformin) were lighter and less adipose than offspring not exposed to GDM. Metformin and insulin-exposed offspring had comparable anthropometric outcomes.
Similar content being viewed by others
Introduction
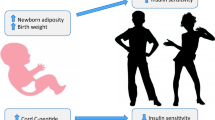
Gestational diabetes mellitus (GDM) is a pregnancy complication characterised by an impaired glucose tolerance (Kaaja & Rönnemaa, 2008). In the United Kingdom (UK), GDM prevalence is approximately 4.4%, a total of 30,625 births each year (National Institute for Health and Care, 2015). GDM poses an important risk for maternal and neonatal health. Foetal exposure to GDM is associated with fat accumulation and growth acceleration in utero, thus, if GDM is left untreated, offspring of women with GDM (OGDM) are more likely to be heavier and more adipose at birth than offspring not exposed to GDM (Logan et al., 2017). In the long term, OGDM have an increased obesity and diabetes risk (Tieu et al., 2017).
Clinical management of GDM aims to maintain maternal glucose levels within healthy ranges, reducing the risk of adverse neonatal outcomes (Tarry-Adkins et al., 2020). Lifestyle changes are initially recommended and if these are insufficient to restore euglycaemia, pharmacotherapy is started (National Institute for Health and Care Excellence (NICE), 2015). Insulin has been historically the first-line pharmacological treatment for GDM. Metformin was introduced as a cheaper and more easily administered alternative to insulin however, it has been associated with safety concerns for the foetus as metformin, unlike insulin, crosses the placenta (Lindsay & Loeken, 2017).
Previous research has investigated the associations between exposure to maternal GDM treatment and offspring birth outcomes. When comparing OGDM to offspring not exposed to GDM, GDM treatment effects remain unclear as recent studies showed that OGDM had lower birth weight and/or were less adipose than offspring not exposed to GDM (Crowther et al., 2005; Landon et al., 2009; Prentice et al., 2019), whilst others described that OGDM were heavier or more adipose than offspring not exposed to GDM despite maternal treatment (Baptiste-Roberts et al., 2012; Prentice et al., 2019). None of those studies however have stratified OGDM by maternal treatment type, it is therefore difficult to determine whether, in comparison to offspring not exposed to GDM, differences in neonatal anthropometric outcomes are specific to the type of treatment prescribed. Further, in the comparison of GDM pharmacological treatment types, it was demonstrated that metformin was a safe alternative to insulin based on evidence of delivery outcomes and neonatal complications (Barrett et al., 2013; Ijäs et al., 2011; Niromanesh et al., 2012; Rowan et al., 2008; Tertti et al., 2013) but in terms of neonatal anthropometry, the evidence to date is largely based on birth weight (Niromanesh et al., 2012; Rowan et al., 2008; Tertti et al., 2008). More accurate evidence regarding neonatal anthropometry, especially in ethnically diverse populations, could however be obtained from circumference measurements and skinfold thicknesses (West et al., 2015).
This study aimed to, in a UK multi-ethnic birth cohort, examine the associations between exposure to maternal GDM treatment and neonatal anthropometry by (1) comparing offspring not exposed to GDM to OGDM exposed to treatment (lifestyle changes advice only or lifestyle changes advice with insulin or metformin) and (2) comparing metformin-exposed offspring to insulin-exposed offspring.
Methods
Study
Born in Bradford (BiB) is a longitudinal prospective birth cohort study conducted between March 2007 and December 2010 at the Bradford Royal Infirmary (Wright et al., 2013). Bradford is one of the UK’s largest cities characterised by high deprivation levels and ethnic diversity (Wright et al., 2013). Recruitment was mainly performed at the time of GDM screening. This was offered between 26 and 28 weeks of pregnancy to all women booked for delivery (80% of women attended the appointment) using the 2 h 75 g oral glucose tolerance test (OGTT) (Wright et al., 2013). Data were collected on 12,453 women, 13,776 pregnancies and 13,858 children. Ethical approval for the study was provided by Bradford Research Ethics Committee (Ref 07/H1302/112). All participants provided informed written consent for the study.
Sample
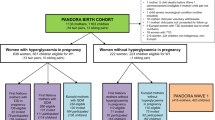
Our sample comprised 9,907 singleton infants with maternal, pregnancy and birth data (Fig. 1).
Inclusion Criteria
If women had more than one singleton pregnancy during the study, only the most recently born infant was considered for inclusion. We included the following: (i) offspring not exposed to GDM, (ii) OGDM with maternal clinical records indicating that lifestyle change advice was received for GDM management (OGDM-Lifestyle), (iii) OGDM with records indicating maternal lifestyle change advice and insulin treatment of GDM (OGDM-Insulin) and (iv) OGDM with medical records specifying maternal lifestyle change advice and metformin treatment of GDM (OGDM-Metformin).
Exclusion Criteria
Stillbirths, twins, and triplets were excluded. Infants born to women with GDM who received any other treatment combination were excluded as the numbers were insufficient to conduct meaningful analyses. Were also excluded infants of women with pre-existing diabetes or where type of GDM treatment was not recorded.
Exposure to Maternal GDM Treatment
We considered four groups: offspring not exposed to GDM, OGDM-Lifestyle, OGDM-Insulin and OGDM-Metformin. GDM was diagnosed using the modified 1999 WHO criteria (fasting glucose concentration ≥ 6.1 mmol/L or 2 h post-load glucose ≥ 7.8 mmol/L) (World Health Organization, 1999). All women with GDM in our sample initially received lifestyle change advice by the clinical team. This consisted of recommendations for changes in dietary habits and a minimum of 30 min walking per day. If glucose targets (fasting plasma glucose: 4.0–5.5 mmol/L; 2 h postprandial: < 7.5 mmol/L) were not achieved by lifestyle changes, supplemental insulin injections alone were prescribed in the first part of the BiB study (04/2007–03/2009). After metformin introduction (04/2009), supplemental insulin injections and metformin tablets (850 mg/L, twice a day) were both pharmacological treatment options for GDM.
Neonatal Outcomes
We examined six neonatal outcomes: birth weight, head, mid-arm and abdominal circumference, and subscapular and triceps skinfold thickness. Data were obtained from maternity hospital notes or electronic health records. Immediately after delivery, birth weight was measured with SECA digital scales. The remaining anthropometric measures were collected within 24 to 72 h following delivery by paediatricians, midwives, and research assistants. Using Lasso-o tapes (Harlow Printing Ltd, South Shields, UK), head circumference was calculated between the most anterior and posterior parts of the head and abdominal circumference was measured at the umbilical level. Subscapular and triceps skinfold thickness measurements were performed on the left side of the new-born with Tanner/Whitehouse Calipers (Holtain Ltd).
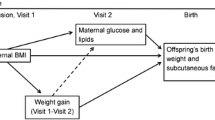
Confounding Variables
Based on recent evidence of maternal characteristics associated with GDM pharmaceutical treatment in the BiB cohort (Martine-Edith et al., 2021) and previous BiB literature (West et al., 2013), we considered maternal body mass index (BMI), height, age at childbirth, ethnicity, parity, fasting and 2 h glucose concentrations at OGTT, smoking during pregnancy as confounding variables. Child sex, gestational age at delivery and route of birth were added to the model as competing exposures (Tennant et al., 2021). Age, parity (0, 1, 2 or 3 + children), smoking (yes/no) and ethnicity (White British (WB), Pakistani, Other) were self-reported by women at recruitment during interviewer-administered questionnaires. Ethnic groups were determined in accordance with the Office of National Statistics guidelines (Office for National Statistics, 2003). Maternal booking BMI was calculated from weight measured at pregnancy booking (10–14 weeks of gestation) using SECA digital scales and height at baseline (26–28 weeks of gestation) measured with Leicester Height Measure. A glucose oxidase method was used to estimate the fasting and 2 h post-load plasma glucose concentrations at OGTT. Gestational age at delivery and route of birth (vaginal or caesarean) were obtained from maternal health records.
Statistical Analysis
Descriptive analysis was used to examine the characteristics of offspring not exposed to GDM, OGDM-Lifestyle, OGDM-Insulin and OGDM-Metformin. Categorical variables were presented as frequencies and percentages. Continuous variables were tested for normality and presented as mean and standard deviation (SD).
We used linear regression to explore the relationships between maternal GDM treatment and offspring birth weight, head, mid-arm and abdominal circumference, and subscapular and triceps skinfold thickness. We assessed these relationships firstly in a whole sample analysis by comparing OGDM-Lifestyle, OGDM-Insulin and OGDM-Metformin to offspring not exposed to GDM. Secondly, in a subsample analysis of OGDM, OGDM-Metformin were compared to OGDM-Insulin. For both analyses we built unadjusted and adjusted models for each outcome.
Lastly, we performed sensitivity analyses. We reproduced the whole sample analysis: (i) in participants with complete data on each outcome, exposure and confounding variable, (ii) stratified by ethnicity, (iii) stratified by route of birth and (iv), in the period after metformin introduction.
Analyses were conducted using Stata/SE software (Stata/SE 15 for Windows; StataCorp, College Station, TX, USA).
Results
Of the 13,858 infants included in the BiB study, 9907 singleton live births met our study inclusion criteria. Our sample comprised 9176 offspring not exposed to GDM, 235 OGDM-Lifestyle, 420 OGDM-Insulin and 76 OGDM-Metformin (Fig. 1).
Descriptive Analysis
Maternal age at childbirth was higher in OGDM than offspring not exposed to GDM (Table 1). Mean maternal BMI was the highest for OGDM-Insulin (29.2 (SD 6.3)) and OGDM-Metformin (29.6 (SD 6.2)). There were more Pakistani women in all OGDM groups than there was in offspring not exposed to GDM. Maternal post-load glucose concentrations at OGTT were higher for OGDM-Insulin (9.2 (SD 1.7)) and OGDM-Metformin (8.9 (SD 1.2)) than for offspring not exposed to GDM (5.4 (SD 1.0)). OGDM were born earlier and lighter than offspring not exposed to GDM. The prevalence of caesarean deliveries was higher among OGDM-Insulin (32.9%), OGDM-Metformin (31.6%), and OGDM-Lifestyle (25.5%) than offspring not exposed to GDM (21.2%).
Whole Sample Analysis: Offspring not Exposed to GDM vs. OGDM Exposed to Treatment
Unadjusted analyses showed that, in comparison to offspring not exposed to GDM, offspring exposure to GDM treatment of any type was associated with lower weight, head, mid-arm and abdominal circumference and higher subscapular skinfold thickness at birth (Table 2).
Birth weight estimates for OGDM-Insulin and OGDM-Metformin in comparison to offspring not exposed to GDM were − 117.2 g (95% CI -173.8, -60.7) and − 200.3 g (− 328.5, − 72.1) respectively, and these were changed to 161.2 g (113.9, 208.4) and 47.6 g (-58.1, 153.3) upon adjustment for gestational age at birth (Online Resource 1). Negative estimates for birth weight and abdominal circumference for all treatment types in comparison to offspring not exposed to GDM were attenuated following adjustments for maternal height and ethnicity (Online Resources 1 and 2). Positive estimates for subscapular skinfold thickness for OGDM-Lifestyle (0.04 mm (-0.1, 0.2)), OGDM-Insulin (0.1 mm (0.02, 0.3)) and OGDM-Metformin (0.01 mm (-0.3, 0.3)) compared to offspring not exposed to GDM became negative after adjusting for maternal glucose concentrations at OGTT (Online Resource 3).
Upon adjustment for all confounding variables, OGDM exposed to treatment of any kind were predicted to have lower weight, mid-arm and abdominal circumference, lower skinfold thicknesses and larger head circumference at birth than offspring not exposed to GDM (Table 2). The standardised coefficients were of similar magnitude for each GDM treatment across the outcomes.
Subsample Analysis: OGDM-Insulin vs. OGDM-Metformin
Unadjusted analyses showed that OGDM-Metformin had smaller mid-arm circumference (-0.4 cm (− 0.7, − 0.1)) and lower triceps skinfold thickness (− 0.3 cm (− 0.6, 0.01)) than OGDM-Insulin (Table 3). The associations between metformin exposure and smaller mid-arm circumference remained negative (− 0.3 cm (− 0.6, − 0.07)) following adjustments for confounding. OGDM-Metformin and OGDM-Insulin did not differ in any other outcomes.
Sensitivity Analyses
The magnitude and direction of the relationships between exposure to GDM treatment in fully adjusted analyses were mostly unchanged by the stratification by maternal ethnicity (Online Resource 4) and route of birth (Online Resource 5). Conducting the same analysis in participants with complete data on all outcomes, exposure and confounding variables did not change the direction of the associations (Online Resource 6). The analysis of OGDM born to women treated after metformin introduction highlighted similar associations with neonatal anthropometric outcomes to those of the main analysis (Online Resource 7).
Discussion
This study showed, in unadjusted analyses, that OGDM exposed to treatment had lower weight and abdominal circumference than offspring not exposed to GDM, which could be partly attributed to a lower gestational age at birth and a greater proportion of Pakistani women in OGDM exposed to treatment. Higher subscapular skinfold thickness at birth in OGDM exposed to treatment compared to offspring not exposed to GDM in unadjusted analyses could be partly attributed to higher glucose concentrations at OGTT in the OGDM groups. Following adjustments for all confounding variables, OGDM exposed to treatment of any kind were predicted to have a lower weight, mid-arm and abdominal circumference, smaller skinfold thicknesses and larger head circumference at birth than offspring not exposed to GDM. The results further demonstrated that OGDM-Metformin were not significantly different from OGDM-Insulin except for a smaller mid-arm circumference in fully adjusted models.
Gestational age at delivery, maternal ethnicity and severity of hyperglycaemia individually contributed to the differences observed between OGDM exposed to treatment and offspring not exposed to GDM. This is consistent with previous research which has shown that after accounting for factors including gestational age, OGDM exposed to treatment (in the study’s most recent cohort) no longer had lower birth weight than offspring not exposed to GDM (Prentice et al., 2019). It is possible that the lower gestational age at birth observed in OGDM exposed to treatment compared to offspring not exposed to GDM may reflect clinical decisions favouring an earlier birth through caesarean section in GDM pregnancies as GDM is associated with risk of macrosomia and shoulder dystocia (Adams et al., 1998; Naylor et al., 1996). No previous studies have been able to clearly show the confounding effect of South Asian ethnicity on the associations between GDM treatment exposure and offspring anthropometry and this may be because of ethnicities that were not White had small sample sizes (Logan et al., 2016) or were broadly categorised into a single group (Prentice et al., 2019). It is hypothesised that, in our study, the large representation of Pakistani women, who have been shown to have offspring with lower birth weight than WB women (West et al., 2010, 2013), in the OGDM groups contributed to the smaller birth weight and abdominal circumference in OGDM compared to offspring not exposed to GDM. Lastly, previous BiB research has shown in unadjusted analyses that the odds of higher offspring adiposity (measured as sum of skinfolds > 90th percentile) at birth increased with maternal glucose levels (Farrar et al., 2015). Our results corroborate this as higher maternal glucose levels at OGTT in the OGDM groups than offspring not exposed to GDM contributed to the observed higher subscapular skinfold thickness in OGDM at birth.
Despite the individual contributions of gestational age at delivery, maternal ethnicity and severity of hyperglycaemia to our results, important differences in anthropometric characteristics remained between OGDM exposed to treatment and offspring not exposed to GDM after adjustments for all covariables simultaneously. Thus, it is likely that GDM treatment itself was associated with lower weight and adiposity in OGDM compared to offspring not exposed to GDM in the cohort. This was demonstrated in offspring of both WB and Pakistani women, and those born from vaginal and caesarean deliveries. Our results are in line with a study conducted in the UK in which a regression discontinuity analysis showed a reduction in birth weight (200 g) and lower odds of large-for-gestational age infants in women diagnosed with GDM and treated for GDM in comparison to women directly below the thresholds for GDM diagnosis (Tennant et al., 2022). It was suggested that the finding of smaller and/or lighter OGDM exposed to treatment than offspring not exposed to GDM in recent studies may be the result of the increasing use of metformin over the years (Prentice et al., 2019), as one of metformin’s mechanisms of action is believed to affect placental glucose transport (Tarry-Adkins et al., 2020). However, this remained uncertain as no studies to date had stratified their analyses by treatment type when comparing OGDM exposed to treatment to offspring not exposed to GDM. Our study therefore provides reassuring evidence for clinical practice that OGDM did not have higher weight or adiposity than offspring not exposed to GDM, regardless of whether women were treated with lifestyle changes advice alone, supplementary insulin or supplementary metformin.
Lastly, this study demonstrated that OGDM-Metformin were comparable to OGDM-Insulin for most anthropometric outcomes. This is in line with previous evidence suggesting that when compared to insulin, metformin treatment is not associated with any significant differences in neonatal anthropometry (Barrett et al., 2013; Ijäs et al., 2011; Rowan et al., 2008; Tertti et al., 2008, 2013). It was however found in this study that OGDM-Metformin had smaller arm circumference than OGDM-Insulin. This may suggest that metformin exposure is associated with less peripheral fat storage which could mean that more fat is stored abdominally (Tarry-Adkins et al., 2020), although no differences were found in abdominal circumference in the current study. Nironamesh et al. (2012) also found in a randomised controlled trial that OGDM-Metformin had smaller arms, in addition to lower birth weight and height, and smaller head and chest circumference at birth. Similarly, other studies have found that OGDM-Metformin had lower birth weight than OGDM-Insulin (Ainuddin et al., 2015; Hamadani et al., 2017). The differences between OGDM-Metformin and OGDM-Insulin were however limited to arm size in our study. Further research is therefore required to establish whether these differences change in childhood. This is especially important as there is evidence that OGDM-Metformin have larger arm circumference and subscapular and biceps skinfold thickness than OGDM-Insulin by 2 years of age, suggesting a healthier pattern of fat storage in early childhood in OGDM-Metformin, as less fat may be stored in the abdomen (Rowan et al., 2011).
Our study had three main strengths. Firstly, these results were based on a large number of OGDM stratified by maternal treatment type which allowed for the assessment of the associations between individual treatment types and neonatal anthropometry, compared to offspring not exposed to GDM. Secondly, evidence has been produced using a variety of anthropometric birth data, allowing for a greater understanding, beyond the differences in birth weight, of the associations between exposure to GDM treatment and offspring adiposity distribution at birth. Lastly, given the differences in the risk of GDM development and neonatal anthropometric characteristics between Pakistani and WB women, the current findings stratified by maternal ethnicity contribute to knowledge by showing in fully adjusted analyses that lower offspring weight and adiposity at birth for OGDM exposed to treatment were observed in both Pakistani and WB groups. Despite these strengths, the study was limited by the relatively low number of women treated with metformin and their offspring, as metformin was introduced in the last two years of the BiB study. Further, there was no data on maternal compliance to treatment which would have had an impact on neonatal outcomes.
To conclude, offspring exposure to GDM treatment of any type in the BiB cohort was associated with lower weight, mid-arm and abdominal circumference and smaller skinfolds at birth compared to offspring not exposed to GDM. There was limited evidence that metformin, compared to insulin, was associated with differences in anthropometric characteristics at birth.
References
Adams, K. M., Li, H., Nelson, R. L., Ogburn, J., Danilenko-Dixon, D. R., Sunkel, D. R., Bofill, J. A., Hill, W. C., Maurus, J. N., Carpenter, R. J., Sandmire, H. F., & Levine, E. M. (1998). Sequelae of unrecognized gestational diabetes. American Journal of Obstetrics and Gynecology. https://doi.org/10.1016/S0002-9378(98)70339-4
Ainuddin, J., Karim, N., Hasan, A. A., & Naqvi, S. A. (2015). Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country. A randomized control trial. Diabetes Research and Clinical Practice,107(2), 290–299. https://doi.org/10.1016/j.diabres.2014.10.001
Baptiste-Roberts, K., Nicholson, W. K., Wang, N. Y., & Brancati, F. L. (2012). Gestational diabetes and subsequent growth patterns of offspring: The national collaborative perinatal project. Maternal and Child Health Journal,16(1), 125–132. https://doi.org/10.1007/s10995-011-0756-2
Barrett, H. L., Gatford, K. L., Houda, C. M., De Blasio, M. J., Mcintyre, H. D., Callaway, L. K., Nitert, M. D., Coat, S., Owens, J. A., Hague, W. M., & Rowan, J. A. (2013). Maternal and neonatal circulating markers ofmetabolic and cardiovascular risk in themetformin in gestational diabetes (mig) trial. Diabetes Care,36(3), 529–536. https://doi.org/10.2337/dc12-1097
Brand, J. S., West, J., Tuffnell, D., Bird, P. K., Wright, J., Tilling, K., & Lawlor, D. A. (2018). Gestational diabetes and ultrasound-assessed fetal growth in south Asian and white European women: Findings from a prospective pregnancy cohort. BMC Medicine,16(1), 1–13. https://doi.org/10.1186/s12916-018-1191-7
Crowther, C. A., Hiller, J. E., Moss, J. R., McPhee, A. J., Jeffries, W. S., & Robinson, J. S. (2005). Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine,352(24), 2477–2486. https://doi.org/10.1056/NEJMoa042973
Farrar, D., Fairley, L., Santorelli, G., Tuffnell, D., Sheldon, T. A., Wright, J., van Overveld, L., & Lawlor, D. A. (2015). Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: Analysis of data from the born in Bradford cohort. The Lancet Diabetes and Endocrinology,3(10), 795–804. https://doi.org/10.1016/S2213-8587(15)00255-7
Hamadani, A., Zahid, S., & Butt, Z. B. (2017). Metformin versus insulin treatment in gestational diabetes in pregnancy and their effects on neonatal birthweight. Pakistan Journal of Medical and Health Sciences,11(3), 914–916.
Ijäs, H., Vääräsmäki, M., Morin-Papunen, L., Keravuo, R., Ebeling, T., Saarela, T., & Raudaskoski, T. (2011). Metformin should be considered in the treatment of gestational diabetes: A prospective randomised study. BJOG: An International Journal of Obstetrics and Gynaecology,118(7), 880–885. https://doi.org/10.1111/j.1471-0528.2010.02763.x
Kaaja, R., & Rönnemaa, T. (2008). Gestational diabetes: Pathogenesis and consequences to mother and offspring. Review of Diabetic Studies,5(4), 194–202. https://doi.org/10.1900/RDS.2008.5.194
Landon, M. B., Spong, C. Y., Thom, E., Carpenter, M. W., Ramin, S. M., Casey, B., Wapner, R. J., Varner, M. W., Rouse, D. J., Thorp, J. M., Jr., Sciscione, A., Catalano, P., Harper, M., Saade, G., Lain, K. Y., Sorokin, Y., Peaceman, A. M., Tolosa, J. E., Anderson, G. B., & Network, E. K. (2009). A multicenter, randomized trial of treatment for mild gestational diabetes. The New England Journal of Medicine,361(14), 1339–1348. https://doi.org/10.1056/NEJMoa0902430
Lindsay, R. S., & Loeken, M. R. (2017). Metformin use in pregnancy: Promises and uncertainties. Diabetologia,60(9), 1612–1619. https://doi.org/10.1007/s00125-017-4351-y
Logan, K. M., Emsley, R. J., Jeffries, S., Andrzejewska, I., Hyde, M. J., Gale, C., Chappell, K., Mandalia, S., Santhakumaran, S., Parkinson, J. R. C., Mills, L., & Modi, N. (2016). Development of early adiposity in infants of mothers with gestational diabetes mellitus. Diabetes Care,39(6), 1045–1051. https://doi.org/10.2337/dc16-0030
Logan, K. M., Gale, C., Hyde, M. J., Santhakumaran, S., & Modi, N. (2017). Diabetes in pregnancy and infant adiposity: Systematic review and meta-analysis. Archives of Disease in Childhood: Fetal and Neonatal Edition,102(1), F65–F72. https://doi.org/10.1136/archdischild-2015-309750
Martine-Edith, G., Johnson, W., Hunsicker, E., Hamer, M., & Petherick, E. S. (2021). Associations between maternal characteristics and pharmaceutical treatment of gestational diabetes: An analysis of the UK Born in Bradford (BiB) cohort study. British Medical Journal Open. https://doi.org/10.1136/bmjopen-2021-053753
National Institute for Health and Care (2015). Diabetes in pregnancy: Management from preconception to the postnatal period. NICE Guidelines [NG3].
National Institute for Health and Care Excellence (NICE) (2015). Diabetes in pregnancy: management from preconception to the postnatal period. NICe, February, 2–65. https://doi.org/978-1-4731-0993-3
Naylor, C. D., Sermer, M., Chen, E., & Sykora, K. (1996). Cesarean delivery in relation to birth weight and gestational glucose tolerance: Pathophysiology or practice style? Journal of the American Medical Association. https://doi.org/10.1001/jama.275.15.1165
Niromanesh, S., Alavi, A., Sharbaf, F. R., Amjadi, N., Moosavi, S., & Akbari, S. (2012). Metformin compared with insulin in the management of gestational diabetes mellitus: A randomized clinical trial. Diabetes Research and Clinical Practice,98(3), 422–429. https://doi.org/10.1016/j.diabres.2012.09.031
Office for National Statistics. (2003). Ethnic group statistics: a guide for the collection and classification of ethnicity data - Office for National Statistics. Office for National Statistics. https://www.ons.gov.uk/methodology/classificationsandstandards/measuringequality/ethnicgroupnationalidentityandreligion
Prentice, P. M., Olga, L., Petry, C. J., Simmons, D., Murphy, H. R., Hughes, I. A., Acerini, C. L., Ong, K. K., & Dunger, D. B. (2019). Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus. Diabetologia,62(11), 1977–1987. https://doi.org/10.1007/s00125-019-4970-6
Rowan, J. A., Hague, W. M., Gao, W., Battin, M. R., & Moore, M. P. (2008). Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine,358(19), 2003–2015. https://doi.org/10.1056/NEJMoa0707193
Rowan, J. A., Rush, E. C., Obolonkin, V., Battin, M., Wouldes, T., & Hague, W. M. (2011). Metformin in gestational diabetes: The offspring Follow-Up (MiG TOFU): Body composition at 2 years of age. Diabetes Care,34(10), 2279–2284. https://doi.org/10.2337/dc11-0660
Tarry-Adkins, J. L., Aiken, C. E., & Ozanne, S. E. (2020). Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLoS Medicine,17(5), 1–23. https://doi.org/10.1371/journal.pmed.1003126
Tennant, P. W. G., Murray, E. J., Arnold, K. F., Berrie, L., Fox, M. P., Gadd, S. C., Harrison, W. J., Keeble, C., Ranker, L. R., Textor, J., Tomova, G. D., Gilthorpe, M. S., & Ellison, G. T. H. (2021). Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. International Journal of Epidemiology. https://doi.org/10.1093/ije/dyaa213
Tennant, P. W. G., Doxford-Hook, E., Flynn, L., Kershaw, K., Goddard, J., & Stacey, T. (2022). Fasting plasma glucose, diagnosis of gestational diabetes and the risk of large for gestational age: A regression discontinuity analysis of routine data. BJOG: An International Journal of Obstetrics and Gynaecology,129(1), 82–89. https://doi.org/10.1111/1471-0528.16906
Tertti, K., Ekblad, U., Vahlberg, T., & Rönnemaa, T. (2008). Comparison of metformin and insulin in the treatment of gestational diabetes: A retrospective, case-control study. Review of Diabetic Studies,5(2), 95–101. https://doi.org/10.1900/RDS.2008.5.95
Tertti, K., Ekblad, U., Koskinen, P., Vahlberg, T., & Rönnemaa, T. (2013). Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obesity and Metabolism,15(3), 246–251. https://doi.org/10.1111/dom.12017
Tieu, J., Aj, M., Ca, C., Middleton, P., & Shepherd, E. (2017). Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD007222.pub4
West, J., Lawlor, D. A., Fairley, L., & Wright, J. (2010). 028 birth size differences between white and pakistani-origin infants by generation: results from the born in Bradford Cohort study. Journal of Epidemiology & Community Health,64(Suppl 1), A11–A11. https://doi.org/10.1136/jech.2010.120956.28
West, J., Lawlor, D. A., Fairley, L., Bhopal, R., Cameron, N., McKinney, P. A., Sattar, N., & Wright, J. (2013). UK-born pakistani-origin infants are relatively more adipose than white British infants: Findings from 8704 mother-offspring pairs in the born-in-Bradford prospective birth cohort. Journal of Epidemiology and Community Health,67(7), 544–551. https://doi.org/10.1136/jech-2012-201891
West, J., Santorelli, G., Lennon, L., O’Connell, K., Corkett, J., Wright, J., Brierley, S., Whincup, P., Cameron, N., & Lawlor, D. A. (2015). Beyond height and weight: A programme of school nurse assessed skinfold measurements from white British and south Asian origin children aged 4–5 years within the born in Bradford cohort study. British Medical Journal Open. https://doi.org/10.1136/bmjopen-2015-008630
World Health Organization (1999). Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus; Geneva: 1999.
Wright, J., Small, N., Raynor, P., Tuffnell, D., Bhopal, R., Cameron, N., Fairley, L., Lawlor, A., Parslow, D., Petherick, R., Pickett, E. S., Waiblinger, K. E., & West, J. (2013). Cohort profile: The born in Bradford multi-ethnic family cohort study. International Journal of Epidemiology,42(4), 978–991. https://doi.org/10.1093/ije/dys112
Acknowledgements
Born in Bradford is only possible because of the enthusiasm and commitment of the children and parents in BiB. We are grateful to all the participants, health professionals, schools and researchers who have made Born in Bradford happen. The authors would also like to thank Dr Donald Whitelaw for providing clinical insight regarding the management and treatment of GDM in the BiB cohort.
Funding
This research was funded by Loughborough University and supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre. E.S.P. and W.J. acknowledge support from the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University, and the University of Leicester. W.J. is supported by a UK Medical Research Council (MRC) New Investigator Research Grant (MR/P023347/1). Born in Bradford received funding from a Wellcome Trust infrastructure grant (WT101597MA) and the National Institute for Health and Care Research (NIHR) under its Collaboration for Applied Health Research and Care (CLAHRC) (IS-CLA-0113-10020), now the Yorkshire and Humber Applied Research Collaboration (NIHR200166). The NIHR Clinical Research Network provided research delivery support for the BiB study. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
GME wrote the manuscript and was responsible for the acquisition, analysis, and interpretation of data. ESP and WJ revised the manuscript and contributed to the acquisition, analysis, and interpretation of data. GME, ESP and WJ are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martine-Edith, G., Johnson, W. & Petherick, E.S. Relationships Between Exposure to Gestational Diabetes Treatment and Neonatal Anthropometry: Evidence from the Born in Bradford (BiB) Cohort. Matern Child Health J 28, 557–566 (2024). https://doi.org/10.1007/s10995-023-03851-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-023-03851-w