Abstract
Microbes are helpful and destructive to human health and other living organisms. Microbes can be eliminated by using antibiotics against them, but their capability to resist regularly encountering antibiotics makes them more injurious. Microbes can adjust and adapt according to the chemicals used against them and become antibiotic resistant. Thus, the requirement for novel antimicrobial compounds increases with time to treat antibiotic-resistant microbes. Fish epidermal mucus encounters various pathogens present in their surrounding environment. It has become a rich source of novel antimicrobial compounds mainly antimicrobial peptides that can be used against various antibiotic-resistant pathogenic microbes. Compounds extracted from epidermal mucus can be used synergistically with other antibiotics or resistance modifying agents to inhibit the growth of resistant microbes. Fishes are consumed as a protein-rich food source worldwide and contribute to the world economy. Diseases in fish cause significant losses in the economic benefits exploited by fishermen and industries based on fisheries products. This paper will review compounds from fish epidermal mucus and their use to control the growth of antibiotic-resistant or non-resistant pathogenic microbes of humans and fishes. So, to increase fisheries’ economic benefits and decrease infections involving resistant microbes.
Similar content being viewed by others
Introduction
Antibiotic resistance occurs when microbes like bacteria and fungi are exposed to a specific antibiotic for an extended period. Microbes develop the potential to defeat the antibiotics used to kill them. Intensive use of antibiotics in veterinary and human healthcare sectors without proper knowledge of the dosage used and unnecessary antimicrobials in agricultural practices promote antibiotic resistance globally (Singhal 2022). Antibiotic-resistant bacteria have been a severe threat in the last three decades as many pathogenic and non-pathogenic microbes have become antibiotic-resistant (Ventola 2015). Nearly 1.3 million deaths were associated with bacterial antimicrobial resistance in 2019 only. 9,29,000 deaths were caused by six antibiotic resistance microbes (Murray et al. 2022). After the emergence of COVID-19, the use of antibiotics spikes abruptly, which is a severe cause of concern in terms of antibiotic resistance ( Mohamad et al. 2022). Antibiotics inhibit the growth and development of microbes by interfering in the synthesis of cell walls and their central dogma pathways to obstruct the synthesis of protein, RNA, and DNA (Neu 1992). Antibiotics also interrupt the division of microbes to inhibit their growth (Bennett et al. 2014). Microbes develop intrinsic factors to cope with antibiotics’ effects through mutation and DNA conjugation. Conjugation of DNA occurs with various microbes to get antibiotic-resistant genes or multiple drug-resistant genes (Munita et al. 2016). There are two ways to tackle resistance in microbes. First is modification in existing antibiotics and formation of different generations of antibiotics. Another way is to explore novel antimicrobial compounds from various biological sources. These compounds can act more effectively on the resistant microbes (Chen et al. 2020).
Emergence of resistance in microbes increases the demand for new antimicrobials. Fishes in aquatic environments are in continuous contact with pathogenic microbes, enabling them to build an immune mechanism to protect themselves. Fishes possess many antimicrobial compounds in different parts of their body as a component of their immune system. The first line of defense against microbes present in water is epidermal mucus. The skin mucus is a physical and chemical barrier to invading microbes. Skin mucus is rich in mucin and other viscous colloids along with many enzymes providing antimicrobial nature to the mucus. Enzymes such as proteases, lectins, AMPs (Antimicrobial peptides), lysozymes, alkaline phosphatases and immunoglobulins are some of the components of mucus that provide antimicrobial nature to the mucus (Dash et al. 2018; Angeles Esteban 2012). Compounds extracted were reported to be effective against many pathogenic, opportunistic and resistant microbes. On further purification and characterization, the compounds could be used commercially in many forms for pharmaceutical uses (Rao et al. 2015).
This review highlights the concern for resistance in microbes and focuses on the way to tackle those resistant microbes using antimicrobial compounds present in fish mucus. Further, the role of compounds extracted from fish mucus in therapeutics against various pathogenic and opportunistic microbes of fishes and humans has also been discussed.
Antibiotics Mechanism of Action and Resistance
Antibiotics are chemicals from organisms like bacteria and fungi that inhibit other microbes’ growth. Chemically these are polypeptides, amino sugars, macrolides and various other compounds (Waksman 1961). Antibiotics act not on the host but on the foreign cells, such as invading microbes. Antibiotic selectivity is based on the difference in the host cell’s physiological and biochemical functions and pathogenic cells (Skold 2011).
Antibiotics have three significant ways to inhibit microbial growth (Neu 1992). Firstly, by inhibiting wall synthesis, antibiotics such as penicillin and cephalosporins act as a pseudo substrate and deacetylase the peptide linkage of the peptidoglycan layer of the microbial cell wall (Spratt and Cromie 1988). Another antibiotic family, vancomycin, ties up with the peptide substrate and prevents the microbial walls’ peptidoglycan layer’s crosslinking. The second mode of inhibition is by blocking the machinery of protein synthesis. Antibiotics such as erythromycin, tetracyclines, and kanamycin interrupt protein synthesis steps (initiation, elongation, and termination). All the steps are highly enzyme-regulated, antibiotics block the active sites of active enzymes and prevent the synthesis of proteins (Fourmy et al. 1996). The third mode of inhibition is by interrupting the DNA replication mechanism, and various enzymes are involved in the replication process. Antibiotics block a specific enzyme’s functioning to prevent DNA replication. Fluoroquinolones are the synthetic antibiotic structure that inhibits topoisomerase’s functioning, and topoisomerase prevents the supercoiling of DNA by cutting, uncoiling and ligating the two DNA strands. Fluoroquinolone blocks the ligating activity of topoisomerase and inhibits DNA replication (Rosen 1990).
Antibiotic resistance was reported after the discovery of antibiotics. Antibiotic resistance is observed in various microbes that resist a specific antibiotic drug commonly used against them. Antibiotic resistance occurs due to the constant exposure of an antibiotic medication to a microbe. Due to penicillin’s intensive use, antibiotic resistance to penicillin was observed during the early 1940s, just a few years after its discovery. In the case of vancomycin, another antibiotic drug, resistance was observed after almost 3 to 4 decades of its discovery, as the use of vancomycin was not that frequent after it was discovered. When exposure to vancomycin increases, resistance against vancomycin is also observed. The number of antibiotics discovered so far was very few, and due to the lack of antibiotic discoveries from the 1960s to the 2020s, reliance on the discovered antibiotics has increased, and resistance to those antibiotics has been observed. This means that after prolonged exposure, microbes adapt and resist the drug that was effective against them earlier (Giedraitiene et al. 2011). In the past two years, after the emergence of the Coronavirus Disease of 2019 (COVID-19), antibiotics have caused a ripple in the antibiotic resistance problem (Rizvi and Ahammad 2022). The primary reasons for antibiotic overuse are the higher rate of infections due to poor healthcare discarding, poor water quality, low immunization, and malnutrition. Easy availability of antibiotics and poor prescriber knowledge or over-the-counter (OTC) purchase of antibiotics also trigger the overuse of antibiotics (Singhal 2022).
Resistance occurs to prolonged exposure and overuse of antibiotics in many ways, such as antibiotic modification, antibiotic efflux, conjugative transferase, and target site modification and hiding. Different antibiotics showed a specific method for the evolution of resistance in them, and resistance comes about by modification in three major classes of antibiotics β-lactam, aminoglycoside and chloramphenicol (Iovine 2013). Antibiotics such as macrolides, tetracyclines and fluoroquinolones are pumped out by efflux pumps on the membrane of resistant bacterial cells (Džidić et al. 2008).
Antimicrobial Compounds from Fish Mucus
Fishes are an essential part of the ecosystem as well as of the economic market. Fishes are used as food, and their products such as, fish liver oil, gelatin, fish albumin, fish protein concentrates, glue, protamine etc., are widely procured worldwide. In 2018-19, the quantity of fish produced was twice that of poultry and three times that of cattle. In the last two decades, the fisheries sector contributed to employment mainly in Asian countries, and the products from fisheries increased 527% from 1990 to 2018 (FAO, 2020). Hence, there is an excellent contribution of fish and aquaculture products to the economy. The benefits of fish in the healthcare sector are well known as fish meat provides Omega 3 fatty acids, vital minerals, and proteins. Apart from this, an unfocused portion of fish is epidermal mucus. Epidermal mucus contains many antimicrobial compounds. Here, we pinpoint those compounds and their application in the healthcare sector.
Fish encounter many pathogens in the aquatic ecosystem, which causes various diseases, leading to a decrease in fish production and, hence, decreasing fish and aquaculture’s economic contribution to the world economy (Béné et al. 2016). In the aquatic ecosystem, fishes’ first line of defense against pathogens is the epidermal layer mucus. The epidermal layer’s mucus composition varies from fish to fish as the mucus-producing cells differ in different fish species. The epidermal layer contains two types of cell stem lines: a proteinaceous cell stem line and a mucus cell stem line. The mucus cell stem line produces three different types of cells: mucus cell or goblet cell, sacchiform cell, and club cell. These three cells produce mucus in different fish species (Shepherd 1993; Zaccone et al. 2001). In studies, Malpighian cells or filament-containing pavement cells were also reportedly involved in epithelial mucus production (Whitear 1970). Mucus is a slippery and slimy layer that covers the fish and protects the fish from invading pathogens. It protects the fish from various pollutants such as surfactants, detergents and heavy metals (Arasu et al. 2013). Mucus also provides the function of respiration, ionic balance and conductance, osmotic regulation, reproduction and excretion (Ingram 1980). Mucus confronts the pathogen present in the aquatic environment and develops various immune responses against them. Mucus contains mucin, a macromolecular glycoprotein gel that reduces friction between fish and the aquatic environment and plays a vital role in immunity (Austin and McIntosh 1998). Mucin is rich in sialic acid, neutral glycoproteins, threonine and proline (Rose and Voynow 2006). Mucus antimicrobial compounds are one of the immune responses generated by the epidermal layer of fishes. Antimicrobial compounds extracted and purified from the mucus contain various classes of compounds such as antimicrobial peptides, pectinase, lysozyme, alkaline phosphatase, cathepsin, lectins and proteases are potent compounds used against diverse pathogens (Arockiaraj et al. 2013). Mucus from some fishes also possesses amphipathic helical peptides like dermaseptin, ceratotoxin, and magainin that act as detergent for the membrane and dissolves the membrane by binding with anionic phospholipids, which results in lysis of cells (Mat Jais et al. 2008; Pickering 1974).
The amount of mucus and biochemical compounds present in mucus is affected by the temperature, pH and salinity of water where the fish inhabit. As observed in gilt-head sea bream, the production of peptides in mucus changes with temperature ranging from (22 − 14) ℃ as the stress caused by the difference in temperature triggers the physiological system of fishes, such as the hypothalamus-pituitary interrenal axis, to produce hormones and excessive mucus (Sanahuja et al. 2019; Iger et al. 1995). Change in pH ranging from (5–9) and salinity stimulates rodlet cells to produce mucus and enzymes such as alkaline phosphatase. Alkaline pH stimulates the mucus cells to produce more mucus, but mucus peptides’ deterioration occurs at alkaline pH. Comparatively, at acidic pH, mucus and alkaline phosphatase production in mucus increases without damage (Iger et al. 1997; Al-Arifa 2011). The variation in enzyme activities is also thought to be related to the thickness of the epidermis, and a thicker epidermis has a more significant frequency of mucus cells producing different enzymes such as alkaline phosphatase, proteases and lysozyme. The mucus composition and the antimicrobial component of mucus vary from species to species as the mucus-producing cell type, surrounding conditions (such as pH, temperature and salinity) tolerance changes as species differ (Iger et al. 1997; Fast 2002). The antimicrobial compounds found in the epidermal mucus of fish can be used against many pathogenic microbes resistant to various antibiotics. Synergism of compounds from mucus and old antibiotics or modifying agents would show effective results against resistive microbes (Gibbons et al. 2003). A description of the major immune components found in fish mucus is now enumerated.
Antimicrobial peptides
AMPs are generally positively charged molecules with a low molecular weight with variations in biochemical properties, chain length, structure and amino acid sequences. AMPs showed activity against all microbes, bacteria, fungi, parasites, and viruses. They were reported as inhibitors of central dogma processes and blocked the synthesis of DNA, RNA and proteins (Patrzykat et al. 2002). First AMP reported from fish epidermal mucus is a 33-residue paradaxin extracted from Pardachirus marmoratus. Paradaxin is an α-helical peptide that effectively inhibits the growth of microbes (Oren and Shai 1996). A 51-residue peptide hipposin extracted from Hippoglossus hippoglossus skin mucus actively inhibits bacterial growth (Birkemo et al. 2003). Cystine-rich AMPs (cathelicidins, liver-expressed AMPs and defensins) were also reported from fish epidermal mucus, which effectively inhibits the growth of gram-negative and gram-positive bacteria (Ángeles Esteban 2012). Some ribosomal AMPs were also reported from fish mucus effective against gram-positive bacteria (Fernandes et al. 2002). H2A and H6 peptides of histone extracted from the epidermal mucus of Oncorhynchus mykiss effectively inhibited bacterial and fungal growth (Fernandes et al. 2002; 2003). Pleurocidin, which is a 25-residue peptide similar to dermaseptin and ceratotoxin, was extracted from Pleuronectes americanus epidermal mucus and tested against various pathogenic microbial strains (Cole et al. 1997). Epinephelus coioides mucus was found to contain epinecidin-1 a 67 amino acid multifunctional AMP reported as an antibacterial, antifungal and anti-tumor agent (Neshani et al. 2019). In a study by Patel et al. (2020), it was observed that different classes of bioactive molecules (small peptides, sphingolipids and fatty acids) having antibacterial properties were purified and characterized from the epidermal mucus of Puntius sophore. Epidermal mucus extract of Takifugu pardalis (puffer fish species) contains a novel 23 amino acid hepcidin type − 2 like peptide that inhibits the growth of gram-negative and gram-positive microbes (Go et al. 2019). Pscidin-4 is a novel peptide of 44 residues in the pscidin family extracted from hybrid striped bass (Morone chrysops female x M. saxatilis male). Pscidin-4 showed activity against 11 fish pathogenic bacteria and 6 human pathogenic bacteria (Noga et al. 2009). So, AMPs from fish mucus could be developed into drugs and antibiotics for fish and human diseases (Rakers et al. 2013).
Lectins
Lectins are carbohydrate-binding proteins or glycoproteins that agglutinate cells and glycoconjugates present in cells. There are various forms of lectin identified from fish skin mucus, including congerins, galectins, intelectin, pufflectin and nattectin etc, showed effective inhibition of microbes. Some Ca+ 2 dependent mannose-binding lectins that bind to the surface of pathogens were also reported from Silururs asotus (Tsutsui et al. 2011). Pufflectins were extracted from pufferfish and reported binding with a parasitic trematode Heterobothrium okamotoi. AJL-2 a novel lectin from eel’s skin mucus was extracted and tested against E coli by Suzuki et al. (2003).
Proteases
Proteases have many types in terms of catalytic activity, fish epidermal mucus possesses serine, cystine and aspartic proteases. All these proteases were reported effective in killing microbes by cleaving the peptide bonds of microbial walls. Proteases activate innate immune components like immunoglobulins, complements or AMPs (Aranishi 1999). A minute amount of anti-protease was also reported from the epidermal mucus of fish Amphiprion clarkii (Wang 2019).
Lysozymes
Lysozymes are polypeptides and a well-known innate immune component reported from many sources like humans, animals, plants, bacteria and viruses. Lysozymes are the most common compound reported in the epidermal mucus of fish by many researchers all over the globe (Ángeles Esteban 2012). The ability of lysozymes to degrade the bond between N-acetyl glucosamine and N-acetylmuramic acid enables them to degrade microbial walls. Lysozymes were also reported to act on chitin of fungal walls (Wu 1999) and on viruses (Lee-Huang et al. 1999). Fish mucus contains c-type and g-type lysozymes, which effectively eliminate the pathogenic and opportunistic microbes (Qasba et al. 1997). Secretions of lysozymes are linked with various factors like stress, wounds and diseases. So, the amount of lysozyme secretion may indicate the health status of the fishes (Dash et al. 2018). Along with mentioned four major classes of antimicrobial compounds present in mucus, some other components such as metabolites, amino acids and fatty acids were also found in the mucus. These components also contribute to antifungal and antibacterial activity of mucus (Uthayakumar et al. 2012).
Fish mucus is a rich source of various novel bioactive molecules and antimicrobials such as AMPs, proteases, fatty acids, lysozymes and galectins. So, fish mucus could be served as a potent source of novel antimicrobial compounds that can help combat antibiotic resistance in microbes.
Use of Antimicrobial Compounds from Fish to Resist the Growth of Antibiotic-resistant Microbes and Aquatic Pathogenic Microbes
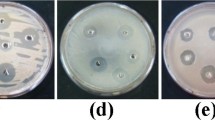
In various studies, mucus antimicrobial activity was observed due to the presence of one or more than one antimicrobial compounds in the mucus of fish. All the compounds present in the mucus can be used against many bacteria, “gram-positive and gram-negative,“ as well as against some fungi. Growth inhibition was observed in studies using mucus of different fish species against various microbes. Three novel antibacterial proteins showed pore-forming activity against microbes such as Staphylococcus aureus and Aeromonas hydrophilla. The proteins were purified from three separate fish species trench, eel and rainbow trout, and the masses of novel antibacterial proteins are 49kDa, 45kDa and 65kDa, respectively. The pore-forming activity was observed using a planar lipid bilayer (PLB) under the influence of applied voltage, when proteins pass through the PLB, their action was measured in terms of conductance (Ebran et al. 2000). Antimicrobial peptides (AMPs) from fish epidermal mucus were reported effective against many pathogens of fish and humans. It was also reported that AMPs from Channa striatus effectively treat wound healing by clotting blood at the wound to prevent bleeding and tissue formation using amino acids and fatty acids from epidermal mucus (Zakaria et al. 2007). AMPs were observed to be useful against several viral infections in fishes, such as viral hemorrhagic septicemia virus in Rainbow trout. It shows effective inhibition by beta-defensins and nervous necrosis virus in Medaka epinecidin-1 (Falco et al. 2007). Another AMP hipposin extracted from Atlantic halibut epidermal mucus showed the inhibition of Enterococcus fecalis, Listeria ivanovii and Staphylococcus epidermis, degrading the microbial walls (Birkemo et al. 2003). Proteases such as cathepsins observed in the epidermal mucus of Japanese eel showed bactericidal activity against pathogenic microbes like Listonella anguillarum, Edwardsiella tarda and Flavobacterium columnare (Aranishi 1999). In Pardachirus marmoratus, 33 residues paradaxin were extracted from skin mucus, these peptides have a helical structure similar to mellitin and showed pore-forming activity against metazoan membranes (Dash et al. 2018; Oren and Shai 1996). A unique epidermal lectin of 35KDa was extracted from Silurus asotus skin mucus and showed bacterial agglutination of Aeromonas salmonicida (Tsutsui et al. 2011).
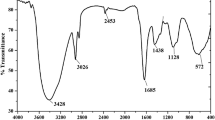
Galectins like congerin were also reported from fish epidermal mucus, which exhibits haemagglutination properties in rabbits, sheep and horses (Shiomi et al. 1989). Lysozymes are the most commonly found antimicrobial compound in fish epidermal mucus. Lysozymes show effective inhibition of many microbes by hydrolyzing the bond between different sugar residues of N-acetyl glucose amine (NAG) and N-acetyl muramic acid (NAM) of microbial walls (Qasba et al. 1997). In some fishes, the compounds in mucus responsible for antimicrobial activity were purified, whereas some compounds must be purified. As in Channa punctatus and Cirrhinus mirgala, antimicrobial activity was observed against nine different bacterial genera, but the compounds responsible for the antimicrobial activity are still unknown (Kuppulakshmi et al. 2008). On the other hand, many compounds were purified from fishes’ mucus, which showed effective microbial growth inhibition, as shown in (Table 1).
Antibiotic-resistant microbe such as Staphylococcus aureus shows resistance against many antibiotics such as Vancomycin, Penicillin, Daptomycin, Tetracyclines, Chloramphenicol, Florfenicol, Methicillin and Oxacillin (Chen et al. 2020; Foster 2017). Fish mucus and antimicrobial components of mucus show effective inhibition of Staphylococcus aureus growth in many studies (Ramesh 2013; Kumari et al. 2019). Inhibition of Methicillin-Resistant Staphylococcus aureus (MRSA) was observed in a study on crude extract, organic extract, and acidic extract of four different freshwater fish species. Acidic extract of Bagrid catfish and Tilapia showed effectual inhibition of MRSA. The other two fishes, Giant snakehead and Stripped snakehead showed poor antimicrobial activity (Rao et al. 2015). In another study, antibiotic resistance of penicillin and chloramphenicol in Streptococcus pneumoniae was observed in 1965 and could be treated through antimicrobial mucus components (Appelbaum 1992). Resistant methicillin was observed in Staphylococcus epidermidis isolated from dental plaque (Tang et al. 2020). The mucus of carp species was found to treat Methicillin-Resistant Staphylococcus epidermidis (MRSE) (Kumari et al. 2019).
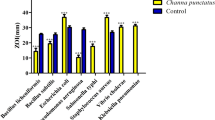
Several microbes are pathogenic, which causes the loss of aquaculture products and affects the health of fishes. It was detected that Cocci, Enterobacteriaceae, Vibrio and Aeromonas proved to be pathogenic to Oncorhynchus mykiss (Rainbow trout). Rainbow trout is native to the Pacific Ocean and edible in around 45 countries worldwide (Diler et al. 2000). Mucus of Hippoglossus hippoglossus showed effective inhibition of these microbes by using a histone H2A derived AMP hipposin. Hipposin disrupts the lipid bilayer glycoproteins, leading to microbial cells’ death (Birkemo et al. 2003). In another study, it was observed that Aeromonas, Pseudomonas, Bacillus and Enterobacteria were pathogenic in Coregonus Albula (staple food of Finland), which were then treated by using mucus of fishes such as Hypophthalmichthys nobilis, Ctenopharyngodon idella and Cyprinus (Kumari et al. 2019; Zmysłowska et al. 2001). Salmonella, Escherichia coli, Klebsiella, Pseudomonas aeruginosa and micrococcus were also found to be pathogenic to Tilapia nilotica (Youssef et al. 1992). These pathogenic microbes present in Tilapia nilotica can be treated using mucus of Hippoglossus fish, Oncorhynchus mykiss and Channa striatus (Birkemo et al. 2003; Ramesh 2013). So, it is proposed that these compounds present in the mucus of fishes could fulfill the requirement of new antimicrobials and can also be considered necessary in fighting antibiotic resistance (Fig. 1).
Synergy of Antimicrobial Compounds to Overcome Resistance
The combination of antibiotics showed synergistic benefits. Newly discovered antimicrobial compounds combined with primitive ones to unleash the true potential of that freshly found antimicrobial compound. Microbes found to be resistive to an antibiotic show effective synergy with an antimicrobial compound, which is also not that practical without the synergistic effect of that resistive antibiotic. Combinations of β-lactams such as penicillin G or ampicillin with aminoglycosidase such as streptomycin or gentamycin showed efficient inhibition of β-lactam-resistant lactobacilli (Bayer et al. 1980). The mechanism behind the effect was not so precise in the case of β -Lactam resistant lactobacilli, but resistance in Staphylococcus aureus against quinolones and antiseptics was well explained by Stermitz et al. (2000). Staphylococcus aureus possesses a multidrug-resistant (MDR) pump NorA which acts as an efflux pump for cationic antimicrobials or quinolones. Several Berberis medicinal plants produce 5-methoxyhydnocarpin (5-MHC), which inhibits the efflux of berberine by binding with NorA MDR pump. Berberine alone hardly showed any inhibition, but in synergy, with (5-MHC) minimum bacterial resistance (MBR) was reduced, and the inhibition by berberine was satisfactory (Stermitz et al. 2000). In the case of Methicillin-Resistant Staphylococcus aureus (MRSA), extract of Lycopus europaeus yielded two new isopimarane diterpenes, namely methyl-1α-acetoxy-7α 14α-dihydroxy-8,15-isopimaradien-18-oate and methyl-1α,14α-diacetoxy-7α-hydroxy-8,15-isopimaradien-18-oate that blocks the MDR pumps of MRSA such as TetK, MsrA and NorA. Tetracycline and erythromycin synergism with these isopimarane diterpenes blocking the efflux pumps show a two-fold reduction in both the antibiotics’ minimum inhibitory concentrations (MICs) (Gibbons et al. 2003).
Conclusion
Various antimicrobial compounds in the fish mucus can be purified and used against pathogenic microbes that adapt or modify to resist antibiotics of any type. Antibiotics are modified slightly (structurally or chemically) when the microbes become resistant to them. For example, generations 1, 2, 3, and 4 antibiotics (Penicillin: Penicillin G, Amoxicillin, Ticarcillin, and Piperacillin, respectively); all these generations of antibiotics have slight differences in their structure. When microbes become resistant to a specific antibiotic compound and its various generations, a new compound with antimicrobial properties is the best-known option. We can observe that in the last 3–4 decades, only 2–3 new antibiotics were discovered, and lack of discovery ultimately promotes resistivity in microbes. So, the resistivity of microbes increases as the availability of antibiotics is more petite. Modifications in antibiotics have been used so far, but it’s not a permanent solution for antibiotic-resistant microbes. Antimicrobial compounds from fish mucus can act as an ideal replacement for antibiotics and can provide novel antimicrobial compounds that effectively prevent resistant microbes’ action. Antimicrobial compounds from fishes are reported to be effective against aquatic pathogens, human pathogens and resistant microbes like MRSA and MRSE. Compounds extracted from mucus can also be used in synergism with old antibiotics and with resistance-modifying agents. Compounds extracted from mucus can also be used in therapeutics and aquaculture to prevent aquatic organisms’ loss due to pathogenic microbes, ultimately enhancing aquaculture products.
Data Availability
Data sharing is not applicable to this review article as no datasets were generated or analyzed during the current study.
References
Al-Arifa N, Mughal MS, Hanif A, Batool A (2011) Effect of alkaline pH on bioactive molecules of epidermal mucus from Labeo rohita (Rahu). Turkish J Biochem 36:29–34
Ángeles Esteban M (2012) An overview of the Immunologyogical defenses in fish skin. Int Sch Res Notices
Appelbaum PC (1992) Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis 15:77–83
Aranishi F, Mano N (2000) Antibacterial cathepsins in different types of ambicoloured japanese flounder skin. Fish Shellfish Immunol 10:87–89
Aranishi F (1999) Possible role for cathepsins B and L in bacteriolysis by japanese eel skin. Fish Shellfish Immunol 8:61–64
Arasu A, Kumaresan V, Sathyamoorthi A, Palanisamy R, Prabha N, Bhatt P, Roy A, Thirumalai MK, Gnanam AJ, Pasupuleti M, Marimuthu K, Arockiaraj J (2013) Fish lily type lectin-1 contains β-prism architecture: immunologyogical characterization. Mol Immunol 56:497–506
Arockiaraj J, Gnanam AJ, Muthukrishnan D, Gudimella R, Milton J, Singh A, Bhassu S (2013) Crustin, a WAP domain containing antimicrobial peptide from freshwater prawn Macrobrachium rosenbergii: immune characterization. Fish Shellfish Immunol 34:109–118
Austin B, McIntosh D (1998) Natural antibacterial compounds on the surface of rainbow trout, Salmo gairdneri. J Fish Diseases 11:275–277
Balasubramanian S, Prakash M, Senthilraja P, Gunasekaran G (2012) Antimicrobial properties of skin mucus from four freshwater cultivable fishes (Catla catla, Hypophthalmichthys molitrix, Labeo rohita, and Ctenopharyngodon idella). Afr J Microbiol Res 6:5110–5120
Bayer AS, Chow AW, Morrison JO, Guze LB (1980) Bactericidal synergy between penicillin or ampicillin and aminoglycosides against antibiotic-tolerant lactobacilli. Antimicrob Agents Chemother 17:359–363
Béné C, Arthur R, Norbury H, Allison EH, Beveridge M, Bush S, Campling L, Leschen, Will, Little D, Squires D, Thilsted SH, Troell M, Williams M (2016) Contribution of fisheries and aquaculture to food security and poverty reduction: assessing the current evidence. World Dev 79:177–196
Bennett JE, Dolin R, Blaser MJ (2014) Mandell, douglas, and bennett’s principles and practice of infectious diseases: 2-volume set, vol 2. Elsevier Health Sciences
Bhatnagar A, Kumari S, Tyor AK (2023) Assessment of bactericidal role of epidermal mucus of Heteropneustes fossilis and Clarias batrachus (asian cat fishes) against pathogenic microbial strains. Aquac Fish 8:50–58
Birkemo GA, Lüders T, Andersen O, Nes I, Nisson-Meyer J (2003) A histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.). Biochim Biophys Acta 1646:207–215
Chen CJ, Huang YC, Shie SS (2020) Evolution of Multi-Resistance to Vancomycin, Daptomycin, and Linezolid in Methicillin-Resistant Staphylococcus aureus Causing Persistent Bacteremia. Front microbiol 11:1414
Cole AM, Weis P, Diamond G (1997) Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. JBC 272:12008–12013
Dash S, Das SK, Samal J, Thatoi HN (2018) Epidermal mucus, a major determinant in fish health: a review. Iran J Vet Res 19:72–81
Diler Ö, Altun S, Çalikuşu F, Diler A (2000) A study on qualitative and quantitative bacterial flora of the rainbow trout (Oncorhynchus mykiss) living in different fish farms. Turkish J Vet Ani Sci 24:251–260
Džidić S, Šušković J, Kos B (2008) Antibiotic resistance mechanisms in bacteria: biochemical and genetic aspects. Food Technol Biotechnol 46:11–21
Ebran N, Julien S, Orange N, Auperin B, Molle G (2000) Isolation and characterization of novel glycoproteins from fish epidermal mucus: correlation between their pore-forming properties and their antibacterial activities. Biochim Biophys Acta Biomembr 1467:271–280
Falco A, Mas V, Tafalla C, Perez L, Coll JM, Estepa A (2007) Dual antiviral activity of human alpha-defensin-1 against viral haemorrhagic septicaemia rhabdovirus (VHSV): inactivation of virus particles and induction of a typeI interferon-related response. Antiviral Res 76:111–112
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome
Fast MD, Sims DE, Burka JF, Mustafa A, Ross NW (2002) Skin morphology and humoral non-specific defense parameters of mucus and plasma in Rainbow trout, Coho and Atlantic salmon. Comp Biochem Physiol A Mol Integr Physiol 132:645–657
Fernandes JM, Smith VJ (2002) A novel antimicrobial function for a ribosomal peptide from rainbow trout skin. Biochem Biophys Res Commun 296:167–171
Fernandes JMO, Saint N, Kemp GD, Smith VJ (2003) Oncorhyncin III: a potent antimicrobial peptide derived from the non-histone chromosomal protein H6 of rainbow trout, Oncorhynchus mykiss. Biochem J 373:621–628
Foster TJ (2017) Antibiotic resistance in Staphylococcus aureus current status and future prospects. FEMS Microbiol Rev 41:430–449
Fourmy D, Recht MI, Blanchard SC, Puglisi JD (1996) Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science 274:1367–1371
Gibbons S, Oluwatuyi M, Veitch NC, Gray AI (2003) Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 62:83–87
Giedraitienė A, Vitkauskienė A, Naginienė R, Pavilonis A (2011) Antibiotic resistance mechanisms of clinically important bacteria. Medicina 47(3):19
Go HJ, Kim CH, Park JB, Kim TY, Lee TK, Oh HY, Park NG (2019) Biochemical and molecular identification of a novel hepcidin type 2-like antimicrobial peptide in the skin mucus of the pufferfish Takifugu pardalis. Fish Shellfish Immunol 93:683–693
Hilles AR, Mahmood S, Waly MI, Kaderi MA, Ahmed QU, Azmi SN, AlAsmari AF, Ali N, Alharbi M, Rauf MA (2022) The therapeutic potential of skin mucus from Asian swamp eel (Monopterus albus): in vivo evaluation and histological evidence. J King Saud Uni Sci 34:102011
Hjelmeland K, Christie M, Raa J (1983) Skin mucus protease from rainbow trout, Salmo gairdneri Richardson, and its biological significance. J Fish Biol 23(1):13–22
Iger Y, Abraham M (1997) Rodlet cells in the epidermis of fish exposed to stressors. Tissue Cell 29:431–438
Iger Y, Balm PH, Jenner HA, Bonga SW (1995) Cortisol induces stress-related changes in the skin of rainbow trout (Oncorhynchus mykiss). General and Comparative Endocrinology. 97:188 – 98
Ingram GI (1980) Substances involved in the natural resistance of fish to infection-a review. J Fish Biol 16:23–60
Iovine NM (2013) Resistance mechanisms in Campylobacter jejuni. Virulence 4:230–240
Kumari S, Tyor AK, Bhatnagar A (2019) Evaluation of the antibacterial activity of skin mucus of three carp species. Int Aquat Res 11:225–239
Kuppulakshmi C, Prakash M, Gunasekaran G, Manimegalai G, Sarojini S (2008) Antibacterial properties of fish mucus from Channa punctatus and Cirrhinus mrigala. Eur Rev Med Pharmacol Sci 12:149–159
Lee-Huang S, Huang PL, Sun Y, Kung HF, Blithe DL, Chen HC (1999) Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc Natl Acad Sci 96:2678–2681
Luders T, Birkemo GA, Nissen-Meyer J, Andersen Nes IF (2005) Proline conformation-dependent antimicrobial activity of a proline-rich histone h1 N-terminal peptide fragment isolated from the skin mucus of Atlantic salmon. Antimicrob Agents Chemother 49:2399–2406
Mat Jais AM, Zakaria ZA, Luo A, Song YX (2008) Antifungal activity of Channa striatus (Haruan) crude extracts. Int J Trop Med 3:43–48
Mohamad IN, Wong CKW, Chew CC, Leong EL, Lee BH, Moh CK, Chenasammy K, Lim SCL, Ker HB (2022) The landscape of antibiotic usage among COVID-19 patients in the early phase of pandemic: a malaysian national perspective. J Pharm Policy Pract 15:1–11
Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Virulence Mechanisms of Bacterial Pathogens 5th edition. 17:481–511
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC et al (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis.The Lancet6736
Nagashima Y, Sendo A, Shimakura K, Shiomi K, Kobayashi T, Kimura B, Fujii T (2001) Antibacterial factors in skin mucus of rabbit fishes. J Fish Biol 58:1761–1765
Najm AA, Azfaralariff A, Dyari HR, Othman BA, Shahid M, Khalili N, Law D, Syed Alwi SS, Fazry S (2021) Anti-breast cancer synthetic peptides derived from the Anabas testudineus skin mucus fractions. Sci rep 11:23182
Neshani A, Zare H, Akbari Eidgahi MR, Khaledi A, Ghazvini K (2019) Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol Toxicol 20:1–11
Neu HC (1992) The crisis in antibiotic resistance. Science 257:1064–1073
Noga EJ, Silphaduang U, Park NG, Seo JK, Stephenson J, Kozlowicz S (2009) Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp Biochem Physiol B Biochem Mol Biol 152:299–305
Oren Z, Shai Y (1996) A class of highly potent antibacterial peptides derived from pardaxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur J Biochem 237:303–310
Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock RE (2002) Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 46:605–614
Patel M, Ashraf MS, Siddiqui AJ, Ashraf SA, Sachidanandan M, Snoussi M, Adnan M, Hadi S (2020) Profiling and role of bioactive molecules from Puntius sophore (Freshwater/brackish fish) skin mucus with its potent antibacterial, antiadhesion, and antibiofilm activities. Biomolecules 10:920
Pickering A (1974) The distribution of mucus cells in the epidermis of the brown trout (Salmo trutta L.) and the arctic charr (salvelins alpinus L). J Fish Biol 6:611–618
Qasba PK, Kumar S, Brew K (1997) Molecular divergence of lysozymes and α-lactalbumin. Crit Rev Biochem Mol Biol 32:255–306
Ranjini S, Muniasamy S, Rameshkumar G, Rajagopal T, Sivakumar T, Ponmanickam P (2020) Bactericidal activity of skin mucus and skin extracts of Catla catla and Channa striatus. Acta Biol Szeged 64:11–16
Ramesh B (2013) Assessment of Antimicrobial peptides from mucus of fish. IJCB 1:5–8
Rakers S, Niklasson L, Steinhagen D, Kruse C, Schauber J, Sundell K, Paus R (2013) Antimicrobial peptides (AMPs) from fish epidermis: perspectives for investigative dermatology. JID133:1140–1149
Rao V, Marimuthu K, Kupusamy T, Rathinam X, Arasu MV, Al-Dhabi NA, Arockiaraj J (2015) Defense properties in the epidermal mucus of different freshwater fish species. Aquac Aquar Conserv Legis 8:184–194
Rizvi SG, Ahammad SZ (2022) COVID-19 and antimicrobial resistance: a cross-study. Sci Total Environ 807:150873
Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86:245–278
Rosen T (1990) 6 the Fluoroquinolone Antibacterial Agents. Prog Med Chem 27:235–295
Sanahuja PI, Fernández-Alacid L, Sánchez Nuño S, Ordóñez-Grande B, Ibarz i Valls A (2019) Chronic cold stress alters the skin mucus interactome in a temperate fish model. Front Physiol 9:1–18
Shabir U, Dar JS, Bhat AH, Ganai BA, Khan IA (2022) Isolation and characterization of β-defensin-like protein 1 from epidermal mucus of fungal infected fish (Cyprinus carpio) and assessment of its antimicrobial potencies. Aquac 23:101056
Shepherd KL (1993) Mucus on the epidermis of fish and its influence on drug delivery. Adv Drug Deliv Rev 11:403–417
Shiomi K, Uematsu H, Yamanaka H, Kikuchi T (1989) Purification and characterization of a galactose-binding lectin from the skin mucus of the conger eel Conger myriaster. Comp Biochem Physiol B. Biochem Mol Biol 92:255
Singhal T (2022) Antimicrobial Resistance: The ‘Other’ Pandemic! IJP 1–7
Skold O (2011) Antibiotics and antibiotic resistance. Wiley, Hoboken, NJ 2011
Spratt BG, Cromie KD (1988) Penicillin-binding proteins of gram-negative bacteria. Clin Infect Dis 10:699–711
Stermitz FR, Lorenz, Tawara N, Zenewicz LA, Lewis K (2000) Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Nat Acad Sci 97:1433–1437
Suzuki Y, Tasumi S, Tsutsui S, Okamoto M, Suetake H (2003) Molecular diversity of skin mucus lectins in fish. Comp Biochem Physiol B Biochem Mol Biol 136:723–730
Tang B, Gong T, Cui Y, Wang L, He C, Lu M, Li Y (2020) Characteristics of oral methicillin-resistant Staphylococcus epidermidis isolated from dental plaque. Int J Oral Sci 12:1–10
Tsutsui S, Komatsu Y, Sugiura T, Araki K, Nakamura O (2011) A unique epidermal mucus lectin identified from catfish (Silurus asotus): first evidence of intelectin in fish skin slime. J Biochem 150:501–514
Uthayakumar V, Ramasubramanian V, Senthilkumar D, Priyadarisini VB, Harikrishnan R (2012) Biochemical characterization, antimicrobial and hemolytic studies on skin mucus of fresh water spiny eel Mastacembelus armatus. Asian Pac J Trop Med 2:S863–S869
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharmacol Ther 40:277–283
Waksman SA (1961) The role of antibiotics in nature. Perspect Biol Med 4:271–278
Wang H, Tang W, Zhang R, Ding S (2019) Analysis of enzyme activity, antibacterial activity, antiparasitic activity and physico-chemical stability of skin mucus derived from Amphiprion clarkii. Fish Shellfish Immunol 86:653–661
Whitear M (1970) The skin surface of bony fishes. J Zool 160:437–454
Wu T, Samaranayake LP, Leung WK, Sullivan PA (1999) Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J Med Microbiol 48:721–730
Youssef H, EL-Timawy AK, Ahmed S (1992) Role of aerobic intestinal pathogens of fresh water fish in transmission of human diseases. J Food Prot 55:739–740
Zaccone G, Kapoor BG, Fasulo S, Ainis L (2001) Structural, histochemical and functional aspects of the epidermis of fishes. Adv Mar Biol 40:253–348
Zakaria ZA, Mat Jais AM, Goh YM, Sulaiman MR, Somchit MN (2007) Amino acid and fatty acid composition of an aqueous extract of Channa striatus (Haruan) that exhibits antinociceptive activity. Cli Exp Pharmacol Physiol 34:198–204
Zmysłowska I, Lewandowska D, Nowakowski T, Kozłowski J (2001) Occurrence of bacteria in water and in vendace (Coregonus albula) during rearing in tanks. Pol J Environ Stud 10:51–56
Acknowledgements
The authors thank the Department of Bioengineering and Biotechnology BIT Mesra for the facilities. The authors also thank Dr. Abhijit Dutta, Ranchi University, for his valuable suggestions. First author acknowledges the University Grants Commission of India (UGC) for Maulana Azad National Fellowship Grant/Award Number: [82 − 27/ 2019 (SA-III)].
Funding
The study was funded by University Grants Commission of India (UGC) for Maulana Azad National Fellowship Grant/Award Number: [82 − 27/ 2019 (SA-III)].
Author information
Authors and Affiliations
Contributions
Ahmed Hussain: Conceptualization, writing the original draft. Shashwati Ghosh Sachan: Supervision, Editing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared that they have no conflict of interest.
Ethical Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussain, A., Sachan, S.G. Fish Epidermal Mucus as a Source of Diverse Therapeutical Compounds. Int J Pept Res Ther 29, 36 (2023). https://doi.org/10.1007/s10989-023-10505-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10505-6