Abstract
Stretching of active muscles leads to a great enhancement of the force developed without increased ATP consumption. The mechanism of force enhancement is still debated and it is not clear if it is due to increased crossbridge strain or to a stretch-induced increase in crossbridge number. The present study, performed on single fibres from tibialis anterior or interosseus muscles of the frog at 5 °C, was aimed at clarifying this point. A striation follower device was used to measure sarcomere length changes. Force was measured during the application of stretches (0.15–3.9 ms duration, 3–7.8 nm per half-sarcomere amplitude) to activated fibres. Small 4 kHz sinusoidal length oscillations, superimposed on the stretches, were used to calculate fibre stiffness with high time resolution. Stiffness increased during the stretch then subsequently decayed, all in parallel with tension. Likewise, during quick releases, stiffness decreased during the release then subsequently recovered in parallel with tension. Comparison of tension and stiffness both during the tetanus rise and also during stretches which doubled tension, imposed on the tetanus rise, indicated that stretch-induced crossbridge recruitment was only about 11 %, suggesting that force enhancement by stretching is mainly due to an increase of individual crossbridge force, whereas crossbridge recruitment plays only a minor role. The accompanying stiffness changes can be explained by non-linearity of myofilament compliance.
Similar content being viewed by others
Introduction
It is well known that stretching of activated skeletal muscles, a condition which occurs frequently in everyday physical activity, increases the force developed by the muscle well above its isometric tetanic value. This phenomenon, in which muscles exert a braking action, occurs with little or no extra expenditure of ATP above that of the isometric state (Curtin and Davies 1973; Månsson 1993; Linari et al. 2003; Loiselle et al. 2010). Maintained force increase by stretching has been investigated by many authors over the years (Katz 1939; Abbott and Aubert 1952; Hill and Howarth 1959; Sugi 1972; Cavagna and Citterio 1974; Edman et al. 1978; Julian and Morgan 1979, 1981; Lombardi and Piazzesi 1990; Stienen et al. 1992; Cavagna 1993; Getz et al. 1998) however, in spite of much effort, its mechanism is still debated (Offer and Ranatunga 2010). Recently published papers suggest that fast stretching of single muscle fibres induces a fast, massive crossbridge recruitment which enhances considerably the braking action of the muscle (Brunello et al. 2007; Fusi et al. 2010). This conclusion was mainly derived from the observation that the force increase during stretch is accompanied by an increase of muscle stiffness, which was attributed to an increase in crossbridge number. This view is in contrast with other reports in the literature suggesting that the maintained force increase following a stretch is mainly due to the increased strain of the elastic components of the sarcomere, crossbridges and myofilaments, whereas crossbridge number increases only by a small fraction above its isometric value (Lombardi and Piazzesi 1990; Månsson 1993; Colombini et al. 2010a, b). The experiments reported here were aimed at clarifying this point. It is usually assumed that changes of sarcomere stiffness, whenever they occur, are due to changes of attached crossbridge number. This is because the compliance of both myofilaments and crossbridges is usually considered to be Hookean, i.e. independent of tension developed by the fibre, but the possible non-linear compliance of these structures cannot be excluded and a consensus has yet to be arrived at (Bagni et al. 1999; Edman 2009; Colombini et al. 2010a; Fusi et al. 2010; Månsson 2010b; Reconditi 2010). If crossbridge or myofilament stiffness increases with tension, it could contribute or even explain entirely the increase of sarcomere stiffness found during stretches without necessarily assuming crossbridge recruitment. Thus, force increase could be accounted for simply by the increased individual crossbridge strain.
We measured fibre stiffness during stretching protocols and, for comparison, releasing protocols. These included maintained stretch, maintained release, and cycles in which the fibre was stretched and then released back to the starting length after a brief interval. In addition, we also measured stiffness during the tetanus rise with and without superimposed stretches. The comparison between stiffness and tension under these two conditions allowed us to calculate the relative number of crossbridges recruited by the stretches. Stiffness was measured using a novel analysis (described in the Methods) which improved time resolution up to 40 μs. The results showed that force transients during and following stretches were accompanied by stiffness changes approximately proportional to force independently of stretching duration in the range 0.15–3.9 ms. Stiffness increased, with no measurable delay, when tension rose during the stretching phase and decreased when, at the end of the stretch, tension relaxed towards the isometric level. At any given tension, stiffness was nearly the same during either the stretching or the relaxation phase. The same finding was also observed both during and after quick releases and also when the stretch was followed within 0.6-2 ms by a quick release which reversed the initial stretch. Stiffness measurements during the tetanus rise indicated that crossbridge recruitment by a stretch that doubled tension was ~11 %, similar to a previous report (Colombini et al. 2010b). Overall our data suggest that force enhancement by stretching is mostly due to the increase of the individual crossbridge force whereas crossbridge recruitment provides only a minor contribution. The accompanying stiffness increase is mostly accounted for by the non-linearity of sarcomere compliance with a minor contribution from the crossbridges recruited.
Methods
Frogs (Rana esculenta) were killed by decapitation followed by destruction of the spinal cord, according to the procedure suggested by the Ethical Committee for Animal Experiments of the University of Florence and the EEC guidelines for animal care of the European Community Council (Directive 86/609/EEC). The number of animals used was minimized by dissecting more than one preparation from each animal. Single intact fibres were dissected from the tibialis anterior muscle (4–6 mm long) or interosseous muscle (3–3.5 mm long). Since we found no differences in the mechanical properties of fibres isolated from these two muscles, data were pooled. Fibres were mounted between the lever arms of a fast force transducer (natural frequency 40–60 kHz) and a fast electromagnetic motor (minimum stretch time 0.1 ms) by means of aluminium foil tendon clips in a thermostatically controlled chamber provided with a glass floor for both ordinary and laser light illumination. Stimuli of alternate polarity, 0.5 ms duration and 1.5 times threshold strength, were applied transversely to the fibre by means of platinum plate electrodes at the minimum frequency necessary to obtain fused tetanic contractions. Sarcomere length was measured using the striation follower device in a fibre segment (1.2–2.5 mm long) selected for striation uniformity in a region as close as possible to the force transducer. Experiments were performed at 5 °C and a resting sarcomere length of about 2.05 μm, so that no change in effective overlap between myofilaments occurred during length steps (Bagni et al. 1988). Ringer’s solution had the following composition (mM): NaCl, 115; KCl, 2.5; CaCl2, 1.8; NaH2PO4, 0.85; Na2HPO4, 2.15. Force, fibre length and sarcomere length signals were recorded using custom-written software (LabView, National Instruments, USA) at sampling rate of up to 200 kHz. Data are expressed as mean ± standard error of the mean (SEM).
Stiffness measurements
Stiffness was measured by applying small sinusoidal length oscillations (l) at 4 kHz frequency to one end of the fibre, superimposed onto ramp stretches, and measuring force oscillations (p) at the other end (Cecchi et al. 1982; 1986). Sarcomere length oscillations (ls) were adjusted to ~0.5 nm per half-sarcomere (hs) peak amplitude. Force and length traces were recorded both with and without superimposed length oscillations (Fig. 1). The subtraction of the two records gave symmetrical force or length oscillations about zero on a flat baseline (bottom traces in Fig. 1A, B) and these traces were used for stiffness measurements. Under no circumstances (stretches or releases) was the phase shift between force and length sinusoids >5°, demonstrating the elastic behaviour of the fibres at this frequency, in agreement with previous reports (Bagni et al. 1990).
Fibre stiffness measurements during a fast stretch applied at tetanus plateau. The figure shows sarcomere length and force changes during and following a fast stretch (duration 0.49 ms; amplitude 5.25 nm hs−1) applied at tetanus plateau with and without superimposed sinusoidal length oscillations (4 kHz frequency, 1 nm hs−1 peak to peak amplitude). Slow (A) and fast (B) time base. Upper 3 traces from the top (sarcomere length signals): stretch plus oscillations; stretch; stretch plus oscillations minus stretch. Lower 3 traces, corresponding force responses
To resolve stiffness changes of a few %, as required in the present experiments, measurements of force and sarcomere length sinusoids must be performed with very high precision. For force oscillations this presents little difficulty, but measuring sinusoidal length oscillations of only 0.1 % sarcomere length peak to peak amplitude precisely is limited by the resolution of the striation follower. In addition, the sarcomere length signal was affected by fast mechanical noise, due to unwanted vertical or lateral fibre displacement during the quick stretches or releases. In some cases where the sarcomere length signal was particularly good, we measured sarcomere stiffness as p/ls. In other cases where sarcomere length noise was not negligible, we measured fibre stiffness as p/l, then corrected for tendon compliance to obtain sarcomere stiffness. The amplitude of symmetrical force oscillations at 4 kHz, shown in Fig. 1B (bottom line), was modulated by changes of fibre stiffness (S) occurring during stretches, according to pI(t) = S(t) lcos(2πft), where pI is the original (in phase) force signal and lcos(2πft) is the sinusoidal length change applied to the fibre. The quadrature force change, pQ(t) = S(t) lsin(2πft), was obtained by performing the Hilbert transform of the force signal. S(t) was then obtained by summing the squares of pI and pQ, finding the square root of the sum, and then dividing by l. The function so obtained describes the envelope of the modulated force signal, representing the fibre or sarcomere stiffness, with an upper frequency response limit close to the carrier frequency of 4 kHz. Although theoretically not necessary, signals were usually low pass FFT filtered at 3.5–3.8 kHz to reduce noise. Later experiments were performed using a different method which potentially measures stiffness changes with no bandwidth limitation. This was achieved by taking two tetanic contractions with length oscillations, one with the sinusoids starting at 0° phase, equivalent to pI, and the other with the length oscillations shifted by 90°, corresponding to pQ, rendering the Hilbert transform unnecessary. S(t) was again recovered as shown above. S(t) obtained by both the Hilbert transform of the force signal and by the simulated transform obtained using phase-shifted force oscillations showed identical time courses.
The mean value of y 0, the minimum instantaneous shortening needed to discharge tetanic tension (a measure of fibre compliance; Ford et al. 1977), measured using sinusoidal length oscillations at tetanus plateau, was 4.00 ± 0.03 nm hs−1 (n = 10), a value consistent with data in the literature. In the same fibres, mean tetanic tension, P 0, was 209 ± 13 kN m−2. The resulting Young modulus was 53 ± 4 MN m−2.
Results
Experiments at the tetanus plateau
Figure 2 shows the averaged time course of force and instantaneous fibre stiffness from 5 fibres, both during and following the application of a maintained fast stretch at the tetanus plateau.
Average relative force and stiffness changes during and following the application of maintained fast stretch at tetanus plateau. Continuous line, relative tension; empty circles, relative fibre stiffness; filled squares, calculated relative sarcomere stiffness. In each fibre, stiffness was group averaged every 20 consecutive measures each made every 5 μs. These values were then averaged in 5 fibres and plotted as mean ± SEM (standard error of the mean, visible only when greater than symbols). For clarity, only part of the data were plotted. Average stretch amplitude, 4.90 ± 0.20 nm hs−1; average stretch duration, 174 ± 8 μs. The slight rounding of the tension peak results from the differences in the ramp duration for the 5 stretches averaged
It can be seen that fibre stiffness both rises and falls synchronously with tension during the stretch, both reaching a peak at the same time then slowly returning towards the isometric level with a similar time course. Average peak stiffness was 1.22 ± 0.02 (n = 5) fold plateau fibre stiffness (S 0) for a tension peak of 1.93 ± 0.08 P 0. Stiffness was reduced to 1.15 ± 0.012 and to 1.11 ± 0.006, 2 and 4 ms after the start of the stretch, respectively. Stiffness data need to be corrected for tendon elasticity to extract sarcomere stiffness. To do this, we investigated the characteristics of tendon elasticity during stretches by comparing sarcomere length changes, measured with the striation follower, with fibre length changes. The results showed that tendon compliance at P 0 accounted for 18.60 ± 1.30 % (n = 8) of total fibre compliance and was slightly non-linear, decreasing at higher tension. Tendon properties can be seen in Fig. 3 where the pooled length-tension relation from 6 fibres is shown. The relation is very close to linear, however, the best fit (continuous line) was obtained with an exponential equation and showed that tendon stiffness increased by 10.4 % as tension increased from P 0 to 2 P 0. It is worthwhile to mention that the length-tension curve of tendon elasticity in the tension interval between P 0 and zero tension showed a greater non-linearity (data not shown), in agreement with previous data (Cecchi et al. 1987; Nocella et al. 2011).
Tendon length-tension relationship during stretches applied at tetanus plateau. Pooled data from 6 fibres. To reduce scattering and highlight any possible shape change, the length-tension relation of each individual fibre was normalized for the length change (stretch amplitude) needed to increase tetanic tension to 1.8 P 0. Stiffness and tension were sampled every 5 μs. The continuous line is the best fit of the equation: y = (−8.7 ± 1.5) + (9.8 ± 1.5) exp(x/13). The slope of the relation (the tendon stiffness) increased by 10.4 % when tension increased from P 0 to 2 P 0 during stretching
Using the tendon characteristics of Fig. 3, we corrected the fibre stiffness data of Fig. 2 to obtain sarcomere stiffness changes. Sarcomere stiffness reached a peak of 1.25 S 0 synchronously with peak tension and decreased to 1.18 and 1.13 after 2 and 4 ms, respectively. These calculated values are shown in Fig. 2 as filled squares. Comparing these values with those of fibre stiffness, it can be concluded that the effect of tendon compliance on fibre stiffness upon stretching are not greater than ~15 %.
Force and stiffness responses to slower stretches of 0.4, 1.3 and 3.75 ms duration are shown in Fig. 4.
Tension and stiffness changes during and following stretches of 0.4 ms (A, B), 1.3 ms (C, D) and 3.75 ms (E, F) duration applied at tetanus plateau. Continuous line, force; empty circles, fibre stiffness. In A, C and E, stiffness and force data are normalized for their respective tetanus plateau values. In B, D and F the same data are shown with different magnification so that changes of tension and stiffness have the same amplitude, to compare the time course of the two parameters. Stretch amplitudes were: 5.6 nm hs−1 in (A), 6.8 nm hs−1 in (C), and 7.6 nm hs−1 in (E). Records in C and D are the average of 2 responses in two different fibres. Each circle represents the group average stiffness measured every 100 μs, in A, B and C, D and 200 μs in E, F. Vertical dashed lines indicate start of fast sampling. Note that stiffness and tension time courses within the first few milliseconds after the start of the stretch are very similar
At any tension, stiffness is very nearly the same during both force rise and relaxation, especially within a few milliseconds after the start of the stretch. In all the experiments, we found that stiffness was slightly but systematically higher than tension at longer delays from the stretch (as shown in Fig. 4).
The dependence of both force and stiffness increases upon the stretching velocity was analysed by measuring the slope of the rising phase of the tension-stiffness plot, which was close to linear in all the 17 fibres examined (mean R square, 0.95 ± 0.0023). An example is shown in Fig. 5A. The dashed line represents the best linear fit of the rising phase (a–b) of the plot.
Normalized tension-stiffness plot of the stretch response of a single fibre (A) and slope of the rising phase of the tension as function of stretch duration (B). A, stretch amplitude, 4.5 nm hs−1, duration, 1.25 ms. Each point represents group-averaged data every 150 μs. a–b force rise during stretch, b–c tension relaxation. Dashed line is the best linear fit of the force rise. Panel B, shows that the slope of the stiffness-tension plot is independent of the stretch duration in the range 0.4–3.75 ms. The continuous line is the best fit of the data obtained with the equation Y = (−0.002 ± 0.0025)x + (0.233 ± 0.007). Faster stretches were not included because the rising phase of the force response was too noisy
Figure 5B shows that the stiffness-tension ratio (the slope of the relation) during the stretches is independent of the stretch duration in the range examined. Its mean value was 0.23 ± 0.0025 (n = 17), indicating that a 100 % increase of tension caused by the stretch was accompanied by a 23 % increase of fibre stiffness. This value is very close to that measured directly during fast stretches and shown in Fig. 2 (~22 % for 93 % tension increase). The independence of the stiffness-tension ratio from the stretching velocity is not expected if the stiffness increase were due to fast recruitment of crossbridges.
For comparison purpose, we measured stiffness changes during and following quick releases applied at tetanus plateau. Figures 6A, B illustrate one of these experiments. Analogously to stretches, fibre stiffness closely parallels tension during its falling phase as well as during the quick tension recovery. Figures 6C, D show a similar force response compared with sarcomere stiffness changes, therefore excluding the effect of tendon compliance. The mechanical noise on the stiffness record is now greater, for the reasons discussed in the Methods, however, it seems clear that sarcomere stiffness and tension have a very similar time course.
Tension and stiffness changes during a quick release applied at tetanus plateau. A and B, tension (continuous line) and fibre stiffness (empty circles) response of a single fibre to a release of 3.8 nm hs−1 amplitude and 120 μs duration. C and D, mean tension (continuous line) and sarcomere stiffness (open squares) changes during a quick release of 3.6 nm hs−1 amplitude and 160 μs duration from 3 fibres. Stiffness data were group-averaged every 40 μs in (A and B) and 80 μs in (C and D). In A and C, stiffness and force data are normalized for their respective tetanus plateau values. In B and D, the same data are shown with different magnification such as that changes of tension and stiffness have the same amplitude to compare better the time course of the two parameters. S 0′, sarcomere stiffness at plateau. Vertical dashed lines indicate start of fast sampling
The release shown in Fig. 6C, D induced an average tension drop of 0.79 ± 0.0025 P 0 (n = 3) and a sarcomere stiffness drop of 0.19 ± 0.010 S 0. Both changes were almost completely reversed during the quick force recovery. If we assume that both myofilament and crossbridge stiffness is linear, this drop of stiffness and its subsequent recovery are necessarily due to crossbridge detachment and re-attachment. Figure 6 shows that between ~4 and 12 ms after the release, stiffness was slightly smaller compared to tension. This was a generalized behaviour and it appears symmetrical to that occurring after quick stretches where stiffness was higher than tension.
Figure 7 shows the superimposed force and stiffness responses to a stretch and a release of the same amplitude and duration applied at the tetanus plateau. Again, the similarity of tension and stiffness time courses in both conditions is apparent.
Superimposed time course of fibre tension and stiffness during a stretch and a release of the same amplitude and duration. Upper 2 traces, sarcomere length; lower traces, tension and stiffness (noisy signals). Stretch and release duration, 1.2 ms; amplitude, 3.46 nm hs−1. Vertical dashed lines indicate start and end of fast sampling. Stiffness trace filtered at 3.5 kHz
Figure 8 shows two examples of force and stiffness changes obtained when the fibre was quickly stretched, held and then released back to the isometric length.
Time course of tension and fibre stiffness changes during two short stretch-release cycles (A and B) of different amplitude and duration applied at tetanus plateau. The stretches were quickly followed by releases which returned the fibre to the pre-stretch length. A, stretch and release amplitude 2.86 nm hs−1, duration 0.18 and 0.13 ms respectively; B, 6.6 nm hs−1, 0.18 and 1 ms. Hold time between stretch and release was 0.36 ms (A) and 2.2 ms (B). Stiffness data filtered at 3.8 kHz
Note that the whole cycle shown in Fig. 8A lasts less than 0.8 ms. Although the oscillations present on the stiffness traces do not allow us to define with precision the changes occurring during the very short hold phase, there is no doubt that in both records the stretch induces a very fast fibre stiffness increase (of ~19 % S 0 in A) synchronous with tension that is quickly reversed during the subsequent release. Since tendon stiffness influences these changes only minimally, as shown in Fig. 2, they must mostly be due to the sarcomere stiffness change. This behaviour was found in all the experiments of this type (n = 11) and was independent of both the delay between stretch and release and also of the stretch amplitude and speed.
Experiments on the tetanus rise and modelling
In a second series of experiments we examined the relationship between tension and stiffness during the isometric tetanus rise with and without the application of fast stretches. This protocol allowed us to compare stiffness data at the same absolute tension under two different conditions: on the tetanus rise, where we know that tension development is caused by the progressive formation of uniform crossbridges (Bagni et al. 2005), and on the tension increase caused by the stretch, where the mechanism is under investigation. At a given tension, tendon and myofilament compliances are the same in both conditions, thus any difference between the two stiffness measures indicate a change of crossbridge stiffness alone.
Figure 9 shows a typical sample record of tension and stiffness changes occurring when the stretch is applied on the tetanus rise at a tension of 0.58 P 0. Analogously to plateau data, stiffness time course follows tension time course, rising during tension rise and falling during tension relaxation with no delay.
Time course of tension and fibre stiffness during a fast stretch applied on the tetanus rise. Upper trace, sarcomere length; intermediate trace, fibre stiffness; lower trace, force. Stretch (2.4 nm hs−1 amplitude and 0.15 ms duration) was applied at a tension of 0.58 P 0 on the tetanus rise. Peak tension was equal to P 0. Stiffness trace was not filtered so as to illustrate the quality of the original signal
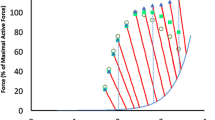
The results of the stiffness-tension measurements on the tetanus rise are shown in Fig. 10 as x–y plot. Filled symbols are stiffness-tension data on the tetanus rise before the stretch and open symbols are those at peak tension at the end of the stretch. It is clear that for the same tension increase, stiffness increases less during the stretches than during the isometric tetanus rise. On average, a stretch inducing a tension increase of 99 ± 5 % increased fibre stiffness, by 17.4 ± 1.1 % compared to the pre-stretch value, whereas the same tension increase during the isometric tetanus rise was accompanied by a stiffness increase of ~54 %, about 3 times greater. A quantitative evaluation of crossbridge recruitment by the stretch was obtained with the model of Colombini et al. 2010a (and relative supplemental material). The model allowed us to calculate the fractional contribution of series and crossbridge compliance to sarcomere compliance by fitting of the tension-stiffness relation on the isometric tetanus rise to the model. It is composed of two springs in parallel, representing the plateau values of the relative compliance of active crossbridges, C b′, inversely proportional to crossbridge number and force (Bagni et al. 2005), and a fixed compliance (independent of tension developed by the fibre), C k, which was attributed to a fraction (~10 % of total crossbridges) of non-force-generating crossbridges whose presence was indicated by the results on crossbridge rupture force (Colombini et al. 2010a). Both springs are in series with another spring representing the series compliance, C s, due to myofilaments. A good fit was obtained when the series compliance was assumed to be non-linear, decreasing at high force (supplemental material of Colombini et al. 2010a). The model is represented by the following equation
Relative stiffness-tension plot during the isometric tetanus rise and during tension increase caused by fast stretches. Stretches of ~0.15 ms duration were applied at various tensions during the tetanus rise. Except for the data indicated by the right and up triangles, stretch amplitude was selected to obtain a peak tension equal to P 0. Filled symbols are stiffness-tension data on the tetanus rise before the stretch and open symbols are those at the end of the stretch. Two equal symbols connected by a dashed line indicate data from an individual fibre. The continuous line represents the best fit of the experimental data points on the tetanus rise to Eq. (1). The thin continuous line represents direct proportionality between tension and stiffness
where S is the total fibre stiffness and h is a factor defining the degree of non-linearity of C s. The value of h was selected to get the appropriate C k fraction of 10 % whereas C s and C b′ were left free to change to obtain the best fit of the stiffness-tension data on the tetanus rise (continuous line and filled points in Fig. 10). To improve the resolution, the fitting was performed on the fibre stiffness-tension data including tendon compliance. However, since tendon compliance depended on tension in the same way as the sarcomere compliance (Colombini et al. 2010a), the fitting was not influenced by its presence. The best fit gave the following factional values: C s, 0.49; C b′, 0.57; C k, 4.98. Total crossbridge compliance, given by the parallel components of 0.57 and 4.98, was 0.51. Considering that our measurements were performed on the whole fibre, to account for the fraction of 0.19 due to tendon compliance, C s, C b′ and C k fractions were reduced proportionally to 0.40, 0.46 and 4.03, respectively. These figures were used to calculate the changes of crossbridge stiffness during stretches. For example, at 0.51 P 0 (filled squares in Fig. 10) the active crossbridge compliance is C b′P 0 /P or 0.46*1.96 or 0.90; taking the parallel element with the fixed fraction of 4.03, we obtain 0.74 as fractional compliance of crossbridges. Now we stretch the fibre and the tension rises from 0.51 P 0 to P 0 at which we measured a relative fibre compliance of 1.26. By subtracting from this value the series and tendon compliances of 0.40 and 0.19 respectively, we obtain the crossbridge compliance at the tension peak of 0.67 whereas prior to the stretch it was 0.74. Thus, the stretch increased the tension by ~96 % and increased crossbridge stiffness from 1/0.74 to 1/0.67 or by 10.4 %. The mean crossbridge stiffness increase induced by stretches which increased mean tension from 0.5 P 0 to P 0, was 11.23 ± 0.029 % (n = 7). This suggests that crossbridges possibly recruited by the stretch are ~11 % of those initially present.
Discussion
In spite of numerous reports present in the literature, the mechanism by which an activated muscle resist forced elongation, developing a greater force than isometric, is still debated (Katz 1939; Abbott and Aubert 1952; Hill and Howarth 1959; Sugi 1972; Cavagna and Citterio 1974; Edman et al. 1978; Julian and Morgan 1979, 1981; Lombardi and Piazzesi 1990; Stienen et al. 1992; Cavagna 1993; Månsson 1993; Getz et al. 1998). In contrast to previous reports (Lombardi and Piazzesi 1990; Colombini et al. 2010a, b), it was suggested recently that fibre stretching induced a very fast recruitment of new myosin motors, companion heads of those already attached, which almost doubled the number of crossbridges attached (Brunello et al. 2007; Fusi et al. 2010). This effect would account for most of the force and stiffness increase found during and immediately after a stretch. Alternatively, force increase by stretching could be explained by the increased strain of the crossbridges and myofilament elasticity, whereas the accompanying stiffness increase could be explained if myofilaments and/or crossbridges have a non-Hookean stiffness, increasing appropriately with tension. Data presented in this paper are better explained by the increased strain of the sarcomere non-linear elasticity rather than by crossbridge recruitment.
Tetanus plateau
When a maintained fast stretch is applied to an activated fibre, tension rises synchronously with the stretch up to a peak tension (phase 1) and then decays to its initial value more slowly during the phase 2 (Ford et al. 1977). Our data showed that these force changes were accompanied by almost proportional stiffness changes which accompanied force without delay, even when the stretch was complete in less than 200 μs. On average, a tension increase of 100 % induced by the stretches, was accompanied by a fibre stiffness increase of ~23 %. Due to the tendon compliance, sarcomere stiffness increased slightly more, reaching 25 % of S 0 (Fig. 2). This peak value decreased by 26 and 46 %, 2 and 4 ms after the start of the stretch, respectively. This result is in contrast with previous data by Fusi et al. (2010) showing that, 2 ms after the stretch, sarcomere stiffness was equal or slightly greater than at tension peak; at 3.65 ms it was only slightly down. The reason of this discrepancy is unknown, but it could be related, at least partially, to the greater time resolution of our stiffness measurements. The first stiffness measurement by Fusi et al. (2010) was taken 625 μs after the start of the stretch, but at this time stiffness was already significantly down (~18 %) from the peak, which occurred ~200 μs after the stretch (Fig. 2). Fusi et al. (2010) attributed the stiffness increase to fast crossbridge recruitment and suggested that these new recruited bridges would detach at the end of the stretch with a time constant of ~10 ms. Hence attached crossbridges and sarcomere stiffness should decrease significantly even 2 ms after the stretch, which seems in contrast with their finding that stiffness remained constant or increased at this time. The fall of stiffness during the relaxation phase shown in Fig. 2 is relatively smaller than the fall of tension. This effect can be explained by (1) the presence of a fraction (~11 %) of crossbridges recruited by the stretch and decaying with a time constant much slower than the fall of force, and (2) by the detachment of strained crossbridges, which reduces force more than stiffness.
The similarity between stiffness and tension time courses can also be seen in response to slower stretches, such as those shown in Fig. 4. We found that stiffness and tension were close to direct proportionality during the stretch as well as during the relaxation phase. All these findings are easily explained by the idea that the tension increase is due to increased strain of crossbridges and that stiffness increase during stretches is due to non-linearity of myofilament and/or crossbridge compliance. The subject of linearity of filament compliance is important, since even a small filament non-linearity could have a significant impact on the number of crossbridge calculated from stiffness changes (Månsson 2010b; Colombini et al. 2010a). Measures of this parameter gave contrasting results in both intact (Bagni et al. 1999; Edman 2009; Colombini et al. 2010a; Fusi et al. 2010; Offer and Ranatunga 2010; Reconditi 2010) and rigor fibres (Podolsky et al. 1982; Higuchi et al. 1995; van der Heide et al. 1997; Fusi et al. 2010). Fusi et al. (2010) found that sarcomere compliance in rigor exhibits a linear behaviour, whereas Podolsky et al. (1982) found that fibre compliance increased at low tension, an effect attributed by Higuchi et al. (1995) to non-linear filament compliance. More recently, data from Irving et al. (2011) in intact fibres, showed that myosin filament compliance is non-linear, increasing substantially at low force. Van der Heide et al. (1997), using fast sinusoidal length oscillations, found that Young’s modulus in rigor fibres decreased at low tension, however, they suggested that non-linearity was probably due to properties of rigor crossbridges rather than myofilaments. This last view seems to be in agreement with recent results from Kaya and Higuchi (2010), showing that myosin molecule elasticity is non-linear. Finally, it is possible that crossbridge elastic properties in rigor may differ from those of crossbridges in intact fibres under physiological conditions, as suggested recently (Chakrabarty et al. 2002).
Figure 4 shows that, at a point longer than ~10 ms after the stretch, there is a phase during which relative stiffness was higher than relative tension. This effect could be attributed to two main reasons: (1) the presence of the static stiffness, a non-crossbridge calcium dependent fibre stiffness, very likely arising from titin filaments, present in frog as well as mammalian fibres (Bagni et al. 2002; 2004; Colombini et al. 2009a; Nocella et al. 2012), and/or by (2) the process of crossbridge detachment–attachment following the length changes (Piazzesi et al. 1997). The contribution of static stiffness to fibre stiffness is very small, a few % of S 0, but it is not negligible in the data of Fig. 4. As regarding the crossbridge kinetics, it has been suggested that stretched crossbridges detach with a time constant of about ~10 ms, while simultaneously new bridges start to substitute for those detached (Piazzesi et al. 1997). The substitution of stretched bridges (generating high force) with unstrained bridges would reduce force more than stiffness, maintaining stiffness relatively higher until the strained bridges have all been substituted. This interpretation is confirmed by the almost symmetrical results from release experiments, in which, a few milliseconds after the release, relative stiffness was lower than tension (Fig. 6). The released crossbridges bear less tension than isometric ones and thus their detachment reduces stiffness more than tension, maintaining stiffness smaller than tension. It should be pointed out that slow stretches, such as those of Fig. 4E, F, are closer to those occurring physiologically during normal everyday activity, in which crossbridge recruitment has been suggested as a major player for the braking action of skeletal muscle during stretching (Brunello et al. 2007).
A quick release induced a synchronous fall of tension and sarcomere stiffness during phase 1 which was recovered during the subsequent phase 2 of the quick force recovery. As during stretches, at any given tension, stiffness was very similar during both phases. If series and crossbridge compliances are both assumed to be linear, stiffness fall induced by the release can only be attributed to fast crossbridge detachment. Data shown in Fig. 6 D indicate a fall of sarcomere stiffness of 19 % during the release. Using the model with linear filament compliance (Colombini et al. 2010a), it can be calculated that this stiffness drop corresponds to a detachment of ~35 % of the crossbridges present. During the force recovery, most of this change is reversed, hence the same amount of crossbridges would re-attach during this phase, and this would occur without any stretch. It would follow that phase 1 and 2 of the force transient cannot be considered exclusively elastic, being greatly affected by crossbridge detachment and recruitment. If true, this point would have a significant impact on our knowledge of crossbridge properties: (1) it would call into question the long standing idea that phase 1 and 2 of the force transient represent the discharge of the tension of the elastic component in series with the crossbridges and the subsequent recovery by the tilting movement of the myosin lever arm (Ford et al. 1977; Colombini et al. 2009b); (2) it would invalidate the T 1 curve (Ford et al. 1977) as a way to measure crossbridge instantaneous elasticity. Alternatively, these findings could be simply explained by assuming that the stiffness of myofilaments or crossbridges is non-linear. Thus, sarcomere stiffness would simply increase and decrease following the tension with no delay.
Figure 8 shows that the quick rise of fibre stiffness induced by a stretch is cancelled out by the quick return of the fibre to its original length. In this case, the recruitment hypothesis would predict a very quick crossbridge recruitment during the stretch, and a similarly quick detachment of the same number of crossbridges during the subsequent quick release. This effect seems unlikely, especially considering that it occurred in all the experiments independent of the stretch or release duration and amplitude used. As with the previous experiments, these results can be simply explained by the stretching of a non-linear sarcomere elasticity.
Another point, in contrast with the crossbridge recruitment hypothesis and consistent with the non-linearity hypothesis, is the observation that the stiffness increase induced by the stretch is independent of the stretch duration in the range 0.4–3.75 ms (Fig. 5B). This is because crossbridges possibly recruited by the stretch would detach with a slower kinetics (~10 ms time constant) compared to the quick tension recovery (<1 ms). Thus, the force response induced by a stretch of 2 ms duration, for example, will be heavily truncated by the quick force recovery whereas fibre stiffness would be much less truncated by the 10 times slower crossbridge detachment. This leads to a tension underestimation which increases the slope of the tension-stiffness relation shown in Fig. 5A. With faster stretches, force responses would increase due to the limited truncation, whereas stiffness would be almost unaffected. The greater force response will decrease the slope of the tension-stiffness relation. Thus, crossbridge recruitment is expected to result in a stiffness-tension relation, whose slope increases for long stretch duration in contrast with the data of Fig. 5B.
Tetanus rise
The experiments with stretches applied on the tetanus rise showed that stretches which doubled tension, applied at ~0.5 P 0, increased the fibre stiffness by ~17.5 %. Data analysis with the model of Colombini et al. (2010a) showed that this fibre stiffness increase was provided by a crossbridge stiffness increase of ~11 %. In principle this increase could be due to either non-linearity of crossbridge compliance or to a small crossbridge recruitment. We prefer the recruitment hypothesis because it is in agreement with our previous data (Colombini et al. 2010b), however we cannot exclude the contribution from a possible non-linearity of crossbridge elasticity. The observation that tension increase during the tetanus rise is due to a progressive attachment of new crossbridges all having the same properties (Bagni et al. 2005) suggests that the conclusion above regarding the tetanus rise could also be extended to the experiments at the tetanus plateau. However, the lack of an independent measure of the fraction of crossbridge compliance for tensions greater than P 0 precluded calculation of the extra crossbridges recruited. The stiffness increase induced by stretches which doubled P 0 was ~23 %, significantly higher than the 17.5 % found on the tetanus rise. This difference is probably due to non-linearity of filament or crossbridge compliances. The recruitment proposed by Fusi et al. (2010), being due to the attachment of the companion myosin head, is expected to be proportional to the number of attached crossbridges. It is worth noticing that during normal physiological activity in vivo, skeletal muscles are usually only partially activated, with unfused tetanic contractions generating submaximal tension, a condition closer to the tetanus rise than to the tetanus plateau.
In conclusion, our data indicate that force increase occurring during and following fast lengthening of active muscles is mainly due to the increase of the mean crossbridge force in agreement with previous suggestion (Månsson 2010a) whereas crossbridge recruitment plays a minor role. The accompanying sarcomere stiffness changes are attributable to non-linearity of myofilament or crossbridges compliance. Similarly, the drop of force during a quick release is mainly due to a reduction of the individual crossbridge force rather than crossbridge detachment.
Contractions during which muscles are stretched by an external force (eccentric contractions) occur practically during any routine movement, performing a braking action and assisting in keeping motions smooth. It is interesting that physical exercises that include exclusively contractions during which muscle shorten and the individual crossbridge force is reduced (Bagni et al. 2005) are less effective in improving muscle performance than those including eccentric contractions, in which individual crossbridge force is increased.
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Bagni MA, Cecchi G, Colomo F, Tesi C (1988) Plateau and descending limb of the sarcomere length-tension relation in short length-clamped segments of frog muscle fibres. J Physiol 401:581–595
Bagni MA, Cecchi G, Colomo F, Poggesi C (1990) Tension and stiffness of frog muscle fibres at full filament overlap. J Muscle Res Cell Motil 11:371–377
Bagni MA, Cecchi G, Colombini B, Colomo F (1999) Sarcomere tension-stiffness relation during the tetanus rise in single frog muscle fibres. J Muscle Res Cell Motil 20:469–476
Bagni MA, Cecchi G, Colombini B, Colomo F (2002) A non-cross-bridge stiffness in activated frog muscle fibers. Biophys J 82:3118–3127
Bagni MA, Colombini B, Geiger P, Berlinguer Palmini R, Cecchi G (2004) Non-cross-bridge calcium-dependent stiffness in frog muscle fibers. Am J Physiol Cell Physiol 286:C1353–C1357
Bagni MA, Cecchi G, Colombini B (2005) Crossbridge properties investigated by fast ramp stretching of activated frog muscle fibres. J Physiol 565:261–268
Brunello E, Reconditi M, Elangovan R, Linari M, Sun YB, Narayanan T, Panine P, Piazzesi G, Irving M, Lombardi V (2007) Skeletal muscle resists stretch by rapid binding of the second motor domain of myosin to actin. Proc Natl Acad Sci USA 104:20114–20119
Cavagna GA (1993) Effect of temperature and velocity of stretching on stress relaxation of contracting frog muscle fibres. J Physiol 462:161–173
Cavagna GA, Citterio G (1974) Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol 239:1–14
Cecchi G, Griffiths PJ, Taylor S (1982) Muscular contraction: kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science 217:70–72
Cecchi G, Griffiths PJ, Taylor S (1986) Stiffness and force in activated frog skeletal muscle fibers. Biophys J 49:437–451
Cecchi G, Colomo F, Lombardi V, Piazzesi G (1987) Stiffness of frog muscle fibres during rise of tension and relaxation in fixed-end or length-clamped tetani. Pflugers Arch 409:39–46
Chakrabarty T, Xiao M, Cooke R, Selvin PR (2002) Holding two heads together: stability of the myosin II rod measured by resonance energy transfer between the heads. Proc Natl Acad Sci USA 99:6011–6016
Colombini B, Benelli G, Nocella M, Musarò A, Cecchi G, Bagni MA (2009a) Mechanical properties of intact single fibres from wild-type and MLC/mIGF-1 transgenic mouse muscle. J Muscle Res Cell Motil 30:199–207
Colombini B, Nocella M, Benelli G, Cecchi G, Griffiths PJ, Bagni MA (2009b) Reversal of the myosin power stroke induced by fast stretching of intact skeletal muscle fibers. Biophys J 97:2922–2929
Colombini B, Nocella M, Bagni MA, Griffiths PJ, Cecchi G (2010a) Is the cross-bridge stiffness proportional to tension during muscle fiber activation? Biophys J 98:2582–2590
Colombini B, Nocella M, Benelli G, Cecchi G, Bagni MA (2010b) Crossbridge properties during force enhancement by slow stretching in single intact frog muscle fibres. J Physiol 585:607–615
Curtin NA, Davies RE (1973) Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harbor Symp Quant Biol 37:619–626
Edman KA (2009) Non-linear myofilament elasticity in frog intact muscle fibres. J Exp Biol 212:1115–1119
Edman KA, Elzinga G, Noble MIM (1978) Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol 281:139–155
Ford LE, Huxley AF, Simmons RM (1977) Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269:441–515
Fusi L, Reconditi M, Linari M, Brunello E, Elangovan R, Lombardi V, Piazzesi G (2010) The mechanism of the resistance to stretch of isometrically contracting single muscle fibres. J Physiol 588:495–510
Getz EB, Cooke R, Lehman SL (1998) Phase transition in force during ramp stretches of skeletal muscle. Biophys J 75:2971–2983
Higuchi H, Yanagida T, Goldman YE (1995) Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69:1000–1010
Hill AV, Howarth JV (1959) The reversal of chemical reactions in contracting muscle during an applied stretch. Proc R Soc Lond B Biol Sci 151:169–193
Irving T, Wu Y, Bekyarova T, Farman GP, Fukuda N, Granzier H (2011) Thick-filament strain and interfilament spacing in passive muscle: effect of titin-based passive tension. Biophys J 100:1499–1508
Julian FJ, Morgan DL (1979) The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol 293:379–392
Julian FJ, Morgan DL (1981) Variation of muscle stiffness with tension during tension transients and constant velocity shortening in the frog. J Physiol 319:193–203
Katz B (1939) The relation between force and speed in muscular contraction. J Physiol 96:45–64
Kaya M, Higuchi H (2010) Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science 329(5992):686–689
Linari M, Woledge RC, Curtin NA (2003) Energy storage during stretch of active single fibres from frog skeletal muscle. J Physiol 548:461–474
Loiselle DS, Tran K, Crampin EJ, Curtin NA (2010) Why has reversal of the actin-myosin cross-bridge cycle not been observed experimentally? J Appl Physiol 108:1465–1471
Lombardi V, Piazzesi G (1990) The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol 431:141–171
Månsson A (1993) Tension transients in skeletal muscle fibres of the frog at varied tonicity of the extracellular medium. J Muscle Res Cell Motil 14:15–25
Månsson A (2010a) Actomyosin-ADP states, interhead cooperativity, and the force-velocity relation of skeletal muscle. Biophys J 98:1237–1246
Månsson A (2010b) Significant impact on muscle mechanics of small nonlinearities in myofilament elasticity. Biophys J 99:1869–1875
Nocella M, Colombini B, Benelli G, Cecchi G, Bagni MA, Bruton J (2011) Force decline during fatigue is due to both a decrease in the force per individual cross-bridge and the number of cross-bridges. J Physiol 589:3371–3381
Nocella M, Colombini B, Bagni MA, Bruton J, Cecchi G (2012) Non-crossbridge calcium-dependent stiffness in slow and fast skeletal fibres from mouse muscle. J Muscle Res Cell Motil 32:403–409
Offer G, Ranatunga KW (2010) Crossbridge and filament compliance in muscle: implications for tension generation and lever arm swing. J Muscle Res Cell Motil 31:245–265
Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V (1997) Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol 498:3–15
Podolsky RJ, Naylor GRS, Arata T (1982) Cross-bridge properties in the rigor state. In: Twarog BM, Levine RJC, Dewey MM (eds) Basic biology of muscles: a comparative approach. Raven, New York
Reconditi M (2010) There is no experimental evidence for non-linear myofilament elasticity in skeletal muscle. J Exp Biol 213:658–659
Stienen GJM, Versteeg PGA, Papp Z, Elzinga G (1992) Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: effects of phosphate and Ca2+. J Physiol 451:503–523
Sugi H (1972) Tension changes during and after stretch in frog muscle fibres. J Physiol 225:237–253
van der Heide U, Ketelaars M, Treijtel BW, de Beer EL, Blangé T (1997) Strain dependence of the elastic properties of force-producing cross-bridges in rigor skeletal muscle. Biophys J 72:814–821
Acknowledgments
This study was supported by the Università di Firenze, Ministero della Ricerca Scientifica (PRIN) and Ente Cassa di Risparmio di Firenze CRF 2010.0256 and CRF 2011.0302.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nocella, M., Bagni, M.A., Cecchi, G. et al. Mechanism of force enhancement during stretching of skeletal muscle fibres investigated by high time-resolved stiffness measurements. J Muscle Res Cell Motil 34, 71–81 (2013). https://doi.org/10.1007/s10974-012-9335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-012-9335-4