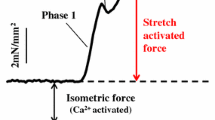
Abstract
Flightin is a myosin rod binding protein that in Drosophila melanogaster is expressed exclusively in the asynchronous indirect flight muscles (IFM). Hyperphosphorylation of flightin coincides with the completion of myofibril assembly and precedes the emergence of flight competency in young adults. To investigate the role of flightin phosphorylation in vivo we generated three flightin null (fln 0) Drosophila strains that express a mutant flightin transgene with two (Thr158, Ser 162), three (Ser139, Ser141, Ser145) or all five potential phosphorylation sites mutated to alanines. These amino acid substitutions result in lower than normal levels of flightin accumulation and transgenic strains that are unable to beat their wings. On two dimensional gels of IFM proteins, the transgenic strain with five mutant sites (fln 5STA) is devoid of all phosphovariants, the transgenic strain with two mutant sites (fln 2TSA) expresses only the two least acidic of the nine phosphovariants, and the transgenic strain with three mutant sites (fln 3SA) expresses all nine phosphovariants, as the wild-type strain. These results suggest that phosphorylation of Thr158 and/or Ser162 is necessary for subsequent phosphorylation of other sites. All three transgenic strains show normal, albeit long, IFM sarcomeres in newly eclosed adults. In contrast, sarcomeres in fully mature fln 5STA and fln 2TSA adults show extensive breakdown while those in fln 3SA are not as disordered. The fiber hypercontraction phenotype that characterizes fln 0 is fully evident in fln 5STA and fln 2TSA but partially rescued in fln 3SA. Mechanics on skinned fibers from newly eclosed flies show alterations in viscous modulus for fln 5STA and fln 2TSA that result in a significant reduction in oscillatory power output. Expression of fln 5STA and fln 2TSA, but not fln 3SA, in a wild-type (fln + /fln +) background resulted in a dominant negative effect manifested as flight impairments and hypercontracted IFM fibers. Our studies indicate that Thr158 and/or Ser162 are (is) indispensable for flightin function and suggest that phosphorylation of one or both residues fulfills an essential role in IFM structural stability and mechanics.







Similar content being viewed by others
Abbreviations
- IFM:
-
Indirect flight muscle
- LMM:
-
Light meromyosin
- MyBP-C:
-
Myosin binding protein C
- 2DE:
-
two-dimensional gel electrophoresis
- RLC:
-
Regulatory light chain
- MALDI-TOF:
-
Matrix assisted laser desorption ionization time-of-flight
- 1DE:
-
One-dimensional gel electrophoresis
- E e :
-
Elastic modulus
- E v :
-
Viscous modulus
References
Arredondo JJ, Mardahl-Dumesnil M, Cripps RM, Cervera M, Bernstein SI (2001) Overexpression of miniparamyosin causes muscle dysfunction and age-dependant myofibril degeneration in the indirect flight muscles of Drosophila melanogaster. J Muscle Res Cell Motil 22:287–299
Ayer G, Vigoreaux JO (2003) Flightin is a myosin rod binding protein. Cell Biochem Biophys 38:41–54
Barton B, Ayer G, Heymann N, Maughan DW, Lehmann FO, Vigoreaux JO (2005) Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J Exp Biol 208:549–560
Cripps RM, Becker KD, Mardahl M, Kronert WA, Hodges D, Bernstein SI (1994) Transformation of Drosophila melanogaster with the wild-type myosin heavy-chain gene: rescue of mutant phenotypes and analysis of defects caused by overexpression. J Cell Biol 126:689–699
Cripps RM, Suggs JA, Bernstein SI (1999) Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J 18:1793–1804
Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND (2001) The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107:631–641
Dickinson MH, Hyatt CJ, Lehmann F-O, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW (1997) Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys J 73:3122–3134
Domingo A, Gonzalez-Jurado J, Maroto M, Diaz C, Vinos J, Carrasco C, Cervera M, Marco R (1998) Troponin-T is a calcium-binding protein in insect muscle: in vivo phosphorylation, muscle-specific isoforms and developmental profile in Drosophila melanogaster. J Muscle Res Cell Motil 19:393–403
Flashman E, Watkins H, Redwood C (2007) Localization of the binding site of the C-terminal domain of cardiac myosin-binding protein-C on the myosin rod. Biochem J 401:97–102
Henkin JA, Maughan DW, Vigoreaux JO (2004) Mutations that affect flightin expression in Drosophila alter the viscoelastic properties of flight muscle fibers. Am J Physiol Cell Physiol 286:C65–C72
Hyatt CJ, Maughan DW (1994) Fourier analysis of wing beat signals: assessing the effects of genetic alterations of flight muscle structure in Diptera. Biophys J 67:1149–1154
Kinumi T, Niwa H, Matsumoto H (2000) Phosphopeptide sequencing by in-source decay spectrum in delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Anal Biochem 277:177–186
Kronert WA, O’Donnell PT, Fieck A, Lawn A, Vigoreaux JO, Sparrow JC, Bernstein SI (1995) Defects in the Drosophila myosin rod permit sarcomere assembly but cause flight muscle degeneration. J Mol Biol 249:111–125
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 270:680–685
Layland J., Solaro RJ, Shah AM (2005) Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66:12–21
Liu H., Miller MS, Swank DM, Kronert WA, Maughan DW, Bernstein SI (2005) Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster. Proc Natl Acad Sci U S A 102:10522–10527
Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M (1998) Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature 395:863–869
Moore JR, Vigoreaux JO, Maughan DW (1999) The Drosophila projectin mutant, bentD, has reduced stretch activation and altered indirect flight muscle kinetics. J Muscle Res Cell Motil 20:797–806
Nongthomba U, Cummins M, Clark S, Vigoreaux JO, Sparrow JC (2003) Suppression of muscle hypercontraction by mutations in the myosin heavy chain gene of Drosophila melanogaster. Genetics 164:209–222
Nongthomba U, Ramachandra NB (1999) A direct screen identifies new flight muscle mutants on the Drosophila second chromosome. Genetics 153:261–274
Obermann WM, Gautel M, Weber K, Furst DO (1997) Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J 16:211–220
Obermann WM, van der Ven FP, Steiner F, Weber K, Furst DO (1998) Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol Biol Cell 9:829–840
Qiu F, Brendel S, Cunha PM, Astola N, Song B, Furlong EE, Leonard KR, Bullard B (2005) Myofilin, a protein in the thick filaments of insect muscle. J Cell Sci 118:1527–1536
Reedy MC, Bullard B, Vigoreaux JO (2000) Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscles. J Cell Biol 151:1483–1499
Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P (1992) In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem 203:173–179
Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn WG 2nd, Klevitsky R, Seidman EC, Seidman GJ, Robbins J (2005) Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res 97:1156–1163
Spradling AC, Rubin MG (1982) Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218:341–347
Sweeney HL, Bowman FB, Stull TJ (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol 264:C1085–C1095
Takano-Ohmuro H, Takahashi S, Hirose G, Maruyama K (1990) Phosphorylated and dephosphorylated myosin light chains of Drosophila fly and larva. Comp Biochem Physiol 95B:171–177
Tohtong R, Yamashita H, Graham M, Haeberle J, Simcox A, Maughan D (1995) Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature 374:650–655
Vigoreaux JO, Hernandez C, Moore J, Ayer G, Maughan D (1998) A genetic deficiency that spans the flightin gene of Drosophila melanogaster affects the ultrastructure and function of the flight muscles. J Exp Biol 201:2033–2044
Vigoreaux JO, Perry ML (1994) Multiple isoelectric variants of flightin in Drosophila stretch-activated muscles are generated by temporally regulated phosphorylations. J Muscle Res Cell Motil 15:607–616
Vigoreaux JO, Saide JD, Pardue ML (1991) Structurally different Drosophila striated muscles utilize distinct variants of Z band-associated proteins. J Muscle Res Cell Motil 12:340–354
Vigoreaux JO, Saide JD, Valgeirsdottir K, Pardue ML (1993) Flightin, a novel myofibrillar protein of Drosophila stretch-activated muscles. J Cell Biol 121:587–598
Vinos J, Domingo A, Marco R, Cervera M (1991) Identification and characterization of Drosophila melanogaster paramyosin. J Mol Biol 220:687–700
Acknowledgements
We are indebted to William Barnes and Mark Miller for help in mechanics, Nicole DeLance for assistance with electron microscopy imaging, and members of the Maughan and Vigoreaux labs for advice and helpful discussions. We thank Hiroyuki Matsumoto for facilitating the mass spectrometry analysis. Supported by NSF grants 0090768 and 0315865 to JOV, and a predoctoral fellowship of the Vermont Genetics Network (BB) through NIH Grant Number 1 P20 RR16462 from the BRIN program of the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barton, B., Ayer, G., Maughan, D.W. et al. Site directed mutagenesis of Drosophila flightin disrupts phosphorylation and impairs flight muscle structure and mechanics. J Muscle Res Cell Motil 28, 219–230 (2007). https://doi.org/10.1007/s10974-007-9120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-007-9120-y