Abstract
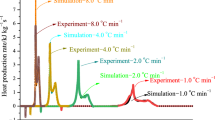
Fireworks are composed of flammable and explosive materials, and during manufacture, transportation, and related processes, these materials are susceptible to static electricity, impact, friction, and explosion degradation, causing fire- and explosion-related accidents. Sorbitan monooleate (SPAN-80) is a widely used emulsifier in emulsion explosives. This study used differential scanning calorimetry and thermogravimetric analysis to characterise the propellant reaction kinetics in fireworks to explore the safety effects of emulsifiers on propellants. Additionally, the intensity of the thermal decomposition reaction of the propellant after the addition of the emulsifier was compared. Simulation models were used to simulate the thermal hazards of propellants and emulsified propellants. The results reveal that the emulsifier affected the decomposition process of the propellant, and the presence of the emulsifier reduced the sensitivity of the propellant. This paper also outlines the thermal hazard parameters of fireworks by using a thermokinetic model, which can serve as a reference for related research.









Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor of the Arrhenius equation (s–1)
- A(α):
-
Pre-exponential factor at conversion (s−1)
- A′(α):
-
Amended pre-exponential factor by a product of A(α) and f(α) (s−1)
- C p :
-
Specific heat capacity (J g−1 K−1)
- E α :
-
Apparent activation energy at specific conversion (kJ mol−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- f(α):
-
Kinetics function (dimensionless)
- i :
-
Component number (dimensionless)
- k i :
-
Reaction rate constant for ith stage (dimensionless)
- n :
-
Unit outer normal on the boundary (dimensionless)
- n 1 , n 2 :
-
Reaction orders of a specific stage (dimensionless)
- \({\text{Q}}_{{\text{i}}}^{\infty }\) :
-
Reaction calorific effect (W)
- R :
-
Gas constant (8.314 J K−1 mol−1)
- r :
-
Reaction rate constant (mol L−1 s−1)
- t :
-
Time (min)
- T :
-
Temperature of sample (K)
- TCL :
-
Time to conversion limit (day)
- T 0 :
-
Apparent exothermic onset temperature (°C)
- T p :
-
Peak temperature (°C)
- TMR :
-
Time to maximum rate (day, min)
- TMR ad :
-
Time to maximum rate under adiabatic conditions (day, min)
- W :
-
Heat generation rate (J s−1)
- w :
-
System external (dimensionless)
- x :
-
Unit outer normal on the boundary (dimensionless)
- z :
-
Autocatalytic constant (dimensionless)
- ∆H d :
-
Heat of decomposition (J g−1)
- α :
-
Conversion degree of a component (dimensionless)
- β :
-
Heating rate (°C min−1)
- λ :
-
Thermal conductivity (W m−1 K−1)
- ρ :
-
Density (kg m−3)
References
Xing Q, Du Z, Zhao L, Yin Q. Study of the environmental humidity influence on the thermal safety of the pyrotechnic compositions. Procedia Eng. 2012;45:552–7.
Surianarayanan M, Sivapirakasam SP, Swaminathan G. Accident data analysis and hazard assessment in fireworks manufacture. Sci Technol Energ Mater. 2008;69:161–8.
Rajathilagam N, Azhagurajan A. Accident analysis in fireworks industries for the past decade in Sivakasi. Int J Res Soc Sci. 2012;2:170–83.
Ajith S, Sivapragasam C, Arumugaprabu V. A review on hazards and their consequences in firework industries. SN Appl Sci. 2018;1:1–6.
Lin W-C, Chen W-C, Shu C-M. Thermal stability evaluation of multiple tubes of fireworks by calorimetry approaches. J Therm Anal Calorim. 2019;138:1–8.
Sivapirakasam SP, Surianarayanan M, Swaminathan G. Hazard assessment for the safe storage, manufacturing and handling of flash compositions. J Loss Prev Process Ind. 2009;22:254–6.
Azhagurajan A, Prakash L, Jeyasubramanian K, Jaya Christa ST. Measurement of MIE and LOC for flash powder mixture containing boron to authenticate safety in firework industries. Measurement. 2020;153:107435.
Schmid P, Bogdal C, Wang Z, Azara V, Haag R, von Arx U. Releases of chlorobenzenes, chlorophenols and dioxins during fireworks. Chemosphere. 2014;114:158–64.
Zhao J-Q, Cheng Y-C, Hou HY, Chen W-C. Applications of intrinsic safety characteristic parameters of propellant dust: commercial multi-tube pyrotechnic hazard assessment. J Loss Prev Process Ind. 2021;69:104381.
Selvakumar N, Azhagurajan A, Suresh A. Experimental analysis on nano scale flash powder composition in fireworks manufacturing. J Therm Anal Calorim. 2012;113:615–21.
Sivapirakasam SP, Harisivasri Phanindra K, Rohin J, Aravind SL. Impact sensitivity of pyrotechnics: a model based on activation energy. Combust Explos Shock Waves. 2020;56:592–600.
Pakkirisamy SV, Mahadevan S, Paramashivan SS, Mandal AB. Adiabatic thermokinetics and process safety of pyrotechnic mixtures. J Therm Anal Calorim. 2011;109:1387–95.
Sridhar VP, Surianarayanan M, Sivapirakasam SP, Mandal AB. Accelerating rate calorimeter studies of water-induced thermal hazards of fireworks tip mixture. J Therm Anal Calorim. 2012;112:1335–41.
Oxley JC, Smith JL, Donnelly M, Porter MR. Fuel oxidizer mixtures: their stabilities and burn characteristics. J Therm Anal Calorim. 2015;121:743–63.
Drapala KP, Auty MAE, Mulvihill DM, Mahony JAO. Influence of emulsifier type on the spray-drying properties of model infant formula emulsions. Food Hydrocoll. 2017;69:56–66.
Sedaghat Doost A, Dewettinck K, Devlieghere F, Van der Meeren P. Influence of non-ionic emulsifier type on the stability of cinnamaldehyde nanoemulsions: a comparison of polysorbate 80 and hydrophobically modified inulin. Food Chem. 2018;258:237–44.
Zheng H, Mao L, Yang J, Zhang C, Miao S, Gao Y. Effect of oil content and emulsifier type on the properties and antioxidant activity of sea buckthorn oil-in-water emulsions. J Food Qual. 2020;2020:1–8.
Wang X, Yuan X, Huang H-j, Leng L, Li H, Peng X, et al. Study on the solubilization capacity of bio-oil in diesel by microemulsion technology with Span80 as surfactant. Fuel Process Technol. 2014;118:141–7.
Farooq A, Shafaghat H, Jae J, Jung S-C, Park Y-K. Enhanced stability of bio-oil and diesel fuel emulsion using Span 80 and Tween 60 emulsifiers. J Environ Manag. 2019;231:694–700.
Koneva AS, Safonova EA, Kondrakhina PS, Vovk MA, Lezov AA, Chernyshev YS, et al. Effect of water content on structural and phase behavior of water-in-oil (n-decane) microemulsion system stabilized by mixed nonionic surfactants Span 80/Tween 80. Colloids Surf A. 2017;518:273–82.
Santini E, Liggieri L, Sacca L, Clausse D, Ravera F. Interfacial rheology of Span 80 adsorbed layers at paraffin oiŒ water interface and correlation with the corresponding emulsion properties. Colloids Surf A. 2007;309:270–9.
Liu H, Qian X, Du Z, Huang P, Liu Z. Thermal explosion model and calculation of sphere fireworks and crackers. J Therm Anal Calorim. 2011;110:1029–36.
Yang Y-P, Huang A-C, Tang Y, Liu Y-C, Wu Z-H, Zhou H-L, et al. Thermal stability analysis of lithium-ion battery electrolytes based on lithium bis(trifluoromethanesulfonyl)imide-lithium difluoro(oxalato)borate dual-salt. Polymers. 2021;13(5):707.
Win SZ, Karin P, Phairote W, Chollacoop N, Saisirirat P, Hanamura K. Investigation of loose contact oxidation kinetic analysis on diesel particulate filter’s wall surface using non-isothermal TGA technique. IOP Conf Ser Mater Sci Eng. 2021;1137(1):012011.
Ahmad MS, Kleme JJ, Alhumade H, Elkamel A, Bokhari A. Thermo-kinetic study to elucidate the bioenergy potential of Maple Leaf Waste (MLW) by pyrolysis, TGA and kinetic modelling. Fuel. 2021;293(3):120349.
Yang Y-P, Jiang J-C, Huang A-C, Tang Y, Liu Y-C, Xie L-J, et al. 3-(Trifluoromethyl)benzoylacetonitrile: a multi-functional safe electrolyte additive for LiNi0.8Co0.1Mn0.1O2 cathode of high voltage lithium-ion battery. Process Saf Environ Prot. 2022;160:80–90.
Lu W, Lin W-C, Huang A-C, Shu C-M. Determination of the ambience duration of lavender essential oil with three perfume fixatives using the thermokinetics approach. J Therm Anal Calorim. 2021;147:1–11.
Liu Y-C, Huang A-C, Tang Y, Huang C-F, Shen Q, Shu C-M, et al. Thermokinetic analysis of the stability of acetic anhydride hydrolysis in isothermal calorimetry techniques. J Therm Anal Calorim. 2021;147:1–9.
Kossoy A, Belochvostov V, Gustin JL. Methodological aspects of the application of adiabatic calorimetry for thermal safety investigation. J Loss Prev Process Ind. 1994;7:397–402.
Liu Y-C, Jiang J-C, Huang A-C, Tang Y, Yang Y-P, Zhou H-L, et al. Hazard assessment of the thermal stability of nitrification by-products by using an advanced kinetic model. Process Saf Environ Prot. 2022;160:91–101.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Gonzales NO, Levin ME, Zimmerman LW. The reactivity of sodium borohydride with various species as characterized by adiabatic calorimetry. J Hazard Mater. 2007;142(3):639–46.
Cao C-R, Chen W-C, Shu C-M. Prediction and assessment of fly-up type of fireworks by thermokinetics model. J Therm Anal Calorim. 2020;142:927–36.
Huang A-C, Huang C-F, Xing Z-X, Jiang J-C, Shu C-M. Thermal hazard assessment of the thermal stability of acne cosmeceutical therapy using advanced calorimetry technology. Process Saf Environ Prot. 2019;131:197–204.
Huang A-C, Huang C-F, Tang Y, Xing Z-X, Jiang J-C. Evaluation of multiple reactions in dilute benzoyl peroxide concentrations with additives using calorimetric technology. J Loss Prev Process Ind. 2021;69:104373.
Cao C-R, Liu S, Das M, Shu C-M. Evaluation for the thermokinetics of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf Environ Prot. 2018;117:426–38.
Liu S, Cao C-R, Lin W-C, Shu C-M. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Cao C-R, Liu S. Thermal hazard characteristic evaluation of two low-temperature-reactive azo compounds under adiabatic process conditions. Process Saf Environ Prot. 2019;130:231–7.
Huang A-C, Li Z-P, Liu Y-C, Tang Y, Huang C-F, Shu C-M, et al. Essential hazard and process safety assessment of para-toluene sulfonic acid through calorimetry and advanced thermokinetics. J Loss Prev Process Ind. 2021;72:104558.
Zhao X, Wang H, Wu W-Q, Zhang J, Chen W-H, Guo Z, et al. Kinetic-parameters-free method of determining autocatalytic decomposition from experimental adiabatic data. Process Saf Environ Prot. 2020;142:250–9.
Zhou H-L, Jiang J-C, Huang A-C, Tang Y, Zhang Y, Huang C-F, et al. Calorimetric evaluation of thermal stability and runaway hazard based on thermokinetic parameters of O, O dimethyl phosphoramidothioate. J Loss Prev Process Ind. 2022;75:104697.
Li Z-P, Jiang J-C, Huang A-C, Tang Y, Miao C-F, Zhai J, et al. Thermal hazard evaluation on spontaneous combustion characteristics of nitrocellulose solution under different atmospheric conditions. Sci Rep. 2021;11:1–13.
Acknowledgements
The authors would like to thank National Key Research Development Program of China (No. 2021YFC3001203), National Natural Science Foundation of China (No. 21927815), and Natural Science Foundation of Jiangsu Higher Education Institutions of China (No. 21KJB620003) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Jiang, JC., Huang, AC. et al. Effect of emulsifiers on the thermal stability of firework propellants. J Therm Anal Calorim 148, 4959–4967 (2023). https://doi.org/10.1007/s10973-022-11473-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11473-7