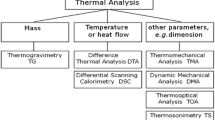
Abstract
Temperature is one of the most frequently used physical parameters, commonly measured but not exactly definable under conditions of non-equilibrium. The first part of this article is devoted to a general understanding of temperature and its measurability. In the second part we deal in detail with temperature as the accessible limit, its physical and operational outlook. For extreme cases of rapid temperature changes we propose an alternative temperature term ‘tempericity.’ Regarding the temperature application we suggest four novel branches of optional alternatives of textbook thermodynamics: thermostatics, thermodynamics, thermotics and thermokinetics. The role of heat transfer is discussed within the validity of the Newton’s cooling law and its impact on the sample heat inertia, which became an important part of a constitutive equation of differential thermal analysis. Variability of temperature data is shown as the function of the procedure applied and sensor positioning. This novel understanding is explained by the analysis of a typical thermoanalytical example of a new field of kinetic phase diagrams. Finally, we point out to a possible correlation of the uncertainty principle with the consequence of measured heat and temperature as well as an operational limit of recordability of temperature. The extended discussion of the literature shows the potential and the pivotal role of JTAC journal in finding new approaches leading to better understanding of thermal analysis.




Similar content being viewed by others
References
Barnett MK. The development of thermometry and the temperature concept. Osiris. 1956;12:269–341.
Quinn STJ. Temperature. London: Academic Press; 1983.
Chang H. Inventing temperature: measurement and scientific progress. Oxford: Oxford University Press; 2007.
Šesták J, Mackenzie RC. The heat/fire concept and its journey from prehistoric time into the third millennium. J Thermal Anal Calorim. 2001;64:129–47.
Šesták J, Mareš JJ. From caloric to stathmograph and polarography. J Thermal Anal Calorim. 2007;88:763.
Holeček M, Mareš JJ, Šesták J. Chapter 3 What is the physical and operational meaning of temperature and its self-measurability during unsteady thermal processes within thermodynamic concepts? In: Šesták J, Hubík P, Mareš JJ, editors. Thermal physics and thermal analysis. New York: Springer; 2017. p. 45–77.
Müller I. A history of thermodynamics. Berlin: Springer; 2007.
Proks I. Evaluation of the knowledge of phase equilibrium: Chapter 1. In: Chvoj Z, Šesták J, Tříska A, editors. Kinetic phase diagrams: nonequilibrium phase transitions. Amsterdam: Elsevier; 1991. p. 1–49.
Šesták J, Hubík P, Mareš JJ. Historical roots and development of thermal analysis and calorimetry: Chapter 21. In: Šesták J, Mareš JJ, Hubík P, editors. Glassy, amorphous and nano-crystalline materials. London: Springer; 2011. p. 347–70.
Proks I. The whole is simpler than its parts: chapters from the history of exact sciences. Bratislava: Veda-Slovak Academy of Sciences; 2012 (in Slovak).
Cardillo P. A history of thermochemistry through the tribulations of its development. J Thermal Anal Calorim. 2002;72:7.
Šesták J. Thermal science and analysis: terms connotation, history, development, and the role of personalities. J Thermal Anal Calorim. 2013;113:1049–54.
Šesták J. Heat, temperature, warmth inspection and calorimetry: Do we know what we measure? In: Invited plenary lecture at the 41. International Czech and Slovak Conference KALSEM, Velké Bílovice 2019.
Kornilov VV, Makarov BI. Measurement of rapidly changing temperatures of conducting solid bodies by means of thermocouples. Meas Tech. 1963;6:849–51.
Merzlyakov M. Integrated circuit thermopile as a new type of temperature sensor in calorimetry. Thermochim Acta. 2003;403:65.
Hes L, Hybil V. Determination of worm-cool feeling of various textiles through thermal absorptivity. Ind J Fibre Text Res. 1991;16:195–9.
Pac MJ, Bueno M, Renner M. Warm-cool feeling relative to tribological properties of fabrics. Text Res J. 2001;71:806–12.
Mangat A, Hes L, Bajzik V. Effect of warm-cool feeling of knitted fabric: a subjective and objective evaluations. AUTEX Res J. 2017;17:95–9.
Price JC. Thermal inertia mapping: a new view of the Earth. J Geophys Res. 1977;82:2582–90.
Williams-Leir G. Effective thermal inertia in relation to normalized heat load. Fire Mater. 1984;8:77–80.
Cracknel AP, Xue Y. Thermal inertia determination: a tutorial review. Int J Remote Sens. 1996;17:431–61.
Wikipedia. http://en.wikipedia.org/wiki/Volumetric_heat_capacity.
Maxwell JC. Theory of heat. London: Longmans Green; 1871.
Zemansky MV. Heat and thermodynamics. Tokyo: McGraw-Hill; 1968.
Holba P. Šesták´s proposal of term “tempericity” for non-equilibrium temperature and modified Tykodi´s thermal science classification with regards to the methods of thermal analysis. J Thermal Anal Calorim. 2017;127:2553–9.
Šesták J, Kratochvíl J. Rational approach to thermodynamic processes and role of constitutive equations. J Thermal Anal. 1973;5:193–9.
Mareš JJ, Hubík P, Šesták J, Špička V, Krištofik J, Stávek J. Phenomenological approach to the caloric theory of heat. Thermochim Acta. 2008;474:16–24.
Šesták J, Mareš JJ, Hubík P, Proks I. Contribution by Lazare and Sadi Carnot to the caloric theory of heat and its stimulation role in alternative thermodynamics. J Thermal Anal Calor. 2009;97:679–83.
Mareš JJ, Šesták J. An attempt at quantum thermal physics. J Thermal Anal Calor. 2005;82:681–6.
Thomson W. (Lord Kelvin of Largs) On the absolute thermometric scale founded on Carnot’s theory of the motive power of heat. Philos Mag. 1848;33:313.
Callendar HL. The caloric theory of heat and Carnot’s principle. Proc Phys Soc Lond. 1911;23:153.
Šesták J. Thermodynamic basis for the theoretical description and correct interpretation of thermoanalytical experiments. Thermochim Acta. 1979;28:197–227.
Šesták J, Šatava V, Wendladt WW. The study of heterogeneous processes by thermal analysis. Thermochim Acta. 1973;13:337–558.
Mareš JJ, Šesták J, Hubík P. Transport Constitutive Relations, Quantum Diffusion and Periodic Reactions: Chapter 14. In: Šesták J, Mareš JJ, Hubík P, editors. Glassy, amorphous and nano-crystalline materials. Berlin: Springer; 2011. p. 227–44.
Šesták J. Measuring, “hotness”, should the sensor’s readings for rapid temperature changes be named “tempericity”? J Thermal Anal Calorim. 2016;125:991–9.
Šesták J. Kinetic phase diagrams as a consequence of radical changing temperature or particle size. J Thermal Anal Calor. 2015;120:129–37.
Adamovsky SA, Minakov AA, Schick C. Scanning microcalorimetry at high cooling rates. Thermochim Acta. 2003;403:55–63.
Minakov A, Morikawa J, Hashimoto T, Huth H, Schick C. Temperature distribution in a thin-film chip utilized for advanced nanocalorimetry. Meas Sci Technol. 2006;17:199–207.
Minakov AA, Schick C. Dynamics of the temperature distribution in ultra-fast thin-film calorimeter sensors. Thermochim Acta. 2015;603:205–17.
Holeček M. Self-measurability in rapid thermal processes. J Thermal Anal Calorim. 2015;120(217–21):38.
Onsager L. Reciprocal relations in irreversible processes. Phys Rev. 1931;37:405.
Tykodi RJ. Thermodynamics of steady state. New York: MacMillan; 1967.
Muschik W. Empirical foundation and axiomatic treatment of non-equilibrium temperature. Arch Ration Mech Anal. 1977;66:379–401.
Exartier R, Peliti L. Measuring effective temperatures in out-of-equilibrium driven Systems. Eur Phys J B. 2000;16:119–26.
Casas-Vazquez J, Jou D. Temperature in non-equilibrium states: a review of open problems and current proposals. Rep Prog Phys. 2003;66:1937–2013.
Šesták J. Thermal analysis, thermokinetics and thermodynamics of phases. Springer, New York; 2021 (In preparation).
Newton I. Philosophiæ naturalis principia mathematica (Mathematical Principles of Natural Philosophy) Londini, jussi Societatus Regiae ac typis Josephi Streater; prostatapud plures bibliopolas, 1687.
Newton I. Scale graduum caloris. Calorum descriptiones and signa Philosophical Trans. 1701;22:824–29.
Holman SW. Calorimetry: methods of cooling correction. Proc Am Acad Arts Sci. 1895/1896;31:245–54.
Fourier JB, Theorie analytique de la chaleur. Paris 1822.
Verbeke S, Audenaert A. Thermal inertia in buildings: a review of impacts across climate and building use. Renew Sustain Energy Rev. 2018;82:2300–18.
Šesták J. Are nonisothermal kinetics fearing historical Newton’s cooling law, or are just afraid of inbuilt complications due to undesirable thermal inertia? J Thermal Anal Calorim. 2018;134:1385–93.
Šesták J. Ignoring heat inertia impairs accuracy of determination of activation energy in thermal analysis. Int J Chem Kinet. 2019;51:74–80.
Speil S. Application of thermal analysis to clays and other aluminous minerals, US Bur Mines, Technical Paper. 1944; R.I. 3764: 1–36.
Vold MJ. Differential thermal analysis. Anal Chem. 1949;21:683–8.
Borchard HJ, Daniels F. The application of DTA to the study of reaction kinetics. J Am Chem Soc. 1957;79:41–6.
Faktor MM, Hanks R. Quantitative application of dynamic differential calorimetry. Trans Faraday Soc. 1967;63:1122–9.
Holba P, Nevřiva M, Šesták J. Analysis of DTA curve and related calculation of kinetic data using computer technique. Thermochim Acta. 1978;23:223–31.
Piloyan GO. Introduction to the theory of thermal analysis. Moskva: Izd. Nauka; 1964 (in Russian).
Garn PD. Thermal analysis of investigation. New York: Academic; 1965.
Schultze D. Differentialthermoanalyze. Berlin: VEB; 1969.
Smykats-Kloss W. Differential thermal analysis. Berlin: Springer; 1974.
Höhne GWH, Hemminger W, Flammersheim HJ. Differential scanning calorimetry. Dortrecht: Springer; 2010.
Vyazovkin S, Koga N, Schick C, editors. Handbook of thermal analysis and calorimetry, recent advances, techniques and applications, vol. 6. Amsterdam: Elsevier; 2018.
Šesták J, Holba P. Heat inertia and temperature gradient in the treatment of DTA peaks. J Thermal Anal Calorim. 2013;113:1633–43.
Holba P, Šesták J. Heat inertia and its role in thermal analysis. J Thermal Anal Calorim. 2015;121:303–7.
Šesták J. The quandary aspects of non-isothermal kinetics beyond the ICTAC kinetic committee recommendations. Thermochim Acta. 2015;611:26–35.
Holba P, Šesták J, Sedmidubský D. Heat transfer and phase transition at DTA experiments: Chapter 5. In: Šesták J, Šimon P, editors. Thermal analysis of micro-, nano- and non-crystalline materials. Berlin: Springer; 2013. p. 99–134.
Holba P, Šesták J. The role of heat transfer and analysis ensuing heat inertia in thermal measurements and its impact to nonisothermal kinetics. Chapter 15. In: Šesták J, Hubík P, Mareš JJ, editors. Thermal physics and thermal analysis. Berlin: Springer; 2017. p. 319–44.
Tian A. Recherches sur le Thermostats; Contribution a l´étude du reglage—thermostats a engeintes multiples. Journal de Chimie-Physique. 1923;20:132–66.
Tian A. Recherches sue la calorimétrie. Généralisation de la méthode de compensation électrique:Microcalorimétrie. J de Chimie-Physiq. 1933;30:665–708.
Calvet E, Prat H. Recent progress in microcalorimetry. Oxford: Pergamon Press; 1963.
Kaisersberger E, Moukhina E. Temperature dependence of the time constants for deconvolution of heat flow curves. Thermochim Acta. 2009;492:101–9.
Sánchez-Rodríguez D, Eloussifi H, Farjas J, Roura P, Dammak M. Thermal gradients in thermal analysis experiments: criterions to prevent inaccuracies when determining sample temperature. Thermochim Acta. 2014;589:37–46.
Chen R, Kirsh Y. Methods for evaluating parameters from thermally stimulated curves: Chapter 6. In: Analysis of thermally stimulated processes. Oxford: Pergamum Press; 1981, pp. 109–110.
Šesták J. Theory and practice of differential thermal analysis, Chapter 12. In: Thermophysical properties of solids: theoretical thermal analysis. Elsevier, Amsterdam; 1984, pp. 303–338; Czech written origin by Academia, Praha (1984) and Russian translation by Mir, Moscow (1988).
Boerio-Goates J, Callen JE. Differential thermal methods: Chapter 8. In: Rossiter BW, Beatzold RC, editors. Determination of thermodynamic properties. Wiley: New York; 1992. p. 621–718.
Šesták J. Science of heat and thermophysical studies: a generalized approaches to thermal analysis. Amsterdam: Elsevier; 2005.
Svoboda H, Šesták J. A new approach to DTA calibration by predetermined amount of Joule heat via rectangular pulses. In: Buzas I, editor. Proceedings of 4th ICTA thermal analysis. Budapest: Akademia Kiado; 1974. p. 726–31.
Holba P, Šesták J. Kinetics with regards to the equilibrium of processes studied at increasing temperatures. Z Phys Chem NF. 1972;80:1–20.
Holba P. Equilibrium background of processes initiated by heating and the Ehrenfest classification of phase transitions: Chapter 2. In: Šesták J, Šimon P, editors. Thermal analysis of micro-, nano- and non-crystalline materials. Springer: Berlin; 2013. p. 29–52.
Holba P. Ehrenfest equations for calorimetry and dilatometry. J Thermal Anal Calorim. 2015;120:175–81.
Mianowski A. Consequences of Holba–Sestak equation. J Thermal Anal Calorim. 2009;96:507–13.
Šesták J. Thermal treatment and analysis: the capability to understand nonequilibrium studies. In: The ICTA/TA invited award lecture, at the 10th ICTA in Salford, UK 1990.
Šesták J. Thermal treatment and analysis: the art of near-equilibrium studies. J Thermal Anal. 1993;40:1293.
Chvoj Z, Šesták J, Tříska J, editors. Kinetic phase diagrams: non-equilibrium phase transitions. Amsterdam: Elsevier; 1991.
Šesták J. Kinetic phase diagrams as an enforced consequence of rapid changing temperature or dismissing particle size: thermodynamic fundamentals and limits: Chapter 5. In: Šesták J, Hubík P, Mareš JJ, editors. Thermal physics and thermal analysis. Berlin: Springer; 2017. p. 109–30.
Šesták J. Composite materials and nanostructured systems: a generalized thermodynamic description. In: Invited plenary lecture, ICCE-22: International conference on composites and nano-engineering, Malta, 2014.
Šesták J. Thermal physics of nanostructured materials: thermodynamic (top–down) and quantum (bottom–up) issues. In: Invited plenary lecture NOM Ostrava 2019, to be published in Materials Today Proceedings.
Smyth HT. Temperature distributions during mineral inversion and its significance in DTA. J Am Cerem Soc. 1951;34:221–4.
Lyon RE, Safronova N, Senese J, Stoliarov SI. Thermokinetic model of sample response in nonisothermal analysis. Thermochim Acta. 2012;545:82.
Šimon P. The single-step approximation: attributes, strong and weak sides employing non-Arrhenius temperature functions. J Thermal Anal Calorim. 2007;88:709–15.
Šimon P, Dubaj T, Cibulková Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature pointing possible correlation of range of measurement. J Thermal Anal Calorim. 2015;120:231–8.
Holba P, Šesták J. Imperfections of Kissinger evaluation method and crystallization kinetics. Glass Phys Chem. 2014;40:486–95.
Šesták J. Is the original Kissinger equation obsolete today—not obsolete the entire non-isothermal kinetics while ignoring heat inertia? J Thermal Anal Calorim. 2014;117:1173–7.
Šesták J, Fiala J, Gavrichev SK. Evaluation of the professional worth of scientific papers, their citation responding and the publication authority. J Thermal Anal Calorim. 2018;131:463–71.
Czarnecki J, Šesták J. The physical kinetics of reversible thermal decomposition: Chapter 17. In: Šesták J, Hubík P, Mareš JJ, editors. Thermal physics and thermal analysis. Berlin: Springer; 2017. p. 363–84.
Dollimore D. We are aware of kinetic problems due to heat transfer, however, we all have our jobs and grants, we just can’t say that we are all wrong and stop working. A privacy note heard during the ESTAC 4 in Jena (formerly DDR) 1987.
White WP. Melting point determination. Am J Sci. 1909;28:453–89.
Acknowledgements
The present work was supported by the CENTEM Project, Reg. No. CZ.1.05/2.1.00/03.0088 that is co-funded from the ERDF as a part of the MEYS—Ministry of Education, Youth and Sports OP RDI Program and in the follow-up sustainability stage supported through the CENTEM PLUS LO 1402. Many thanks are due to Dr. Jerry Czarnecki (formerly with Chan Instruments, USA) who has worked with me for years sharing his original thermoanalytical solution and also Prof. Peter Šimon (Slovak Technical University in Bratislava) who has been cooperating for long time while solving various thermokinetic problems. Kind attention by deceased Dr. Pavel Holba (formerly with the Westbohemian University in Plzeň) is belatedly appreciated as well as friendship of my Japanese collaborator who became a part of our family, Prof. Nabuyoshi Koga, President of ICTAC (Hiroshima University, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Šesták, J. Do we really know what temperature is: from Newton’s cooling law to an improved understanding of thermal analysis. J Therm Anal Calorim 142, 913–926 (2020). https://doi.org/10.1007/s10973-019-09149-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09149-w