Abstract
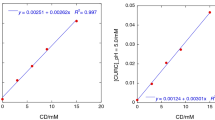
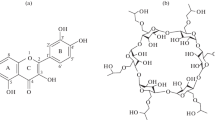
Quercetin (QCT) is a flavonoid possessing many activities, such as neuro-/cardioprotective, anti-inflammatory and anticancer, but its pharmacological application is severely curtailed by its low water solubility and in vivo bioavailability. The formation of a QCT–hydroxypropyl-β-cyclodextrin (HPβCD) host–guest complex is promising to improve QCT therapeutic potential. Therefore, here the heat effects of HPβCD solutions with QCT solutions in water–ethanol solvents at different concentrations were studied by calorimetric titration, and the stability of molecular complexes was assessed by UV–Vis spectrophotometry. Calorimetric titrations revealed the formation of a QCT/HPβCD host–guest complex with a stoichiometric ratio of 1:1 in X(EtOH) = 0.00, 0.05 and 0.10 molar fractions of solvents at pH = 7.0 and pH = 8.1. Thermodynamic parameters of the complex formation reaction (lgK; ΔrH; TΔrS) were obtained in these experimental conditions. Differently, no complex formation was noticed in water–ethanol mixed solvent when ethanol volume fraction exceeded 0.2 at neutral and alkaline pH, as well as a volume fraction higher than 0.1 at acidic pH. Furthermore, the results of differential scanning calorimetry tests run on dried HPβCD after dissolution in hydroalcoholic solutions indicated that ethanol and water compete for the complexation within the hydrophobic cavity of HPβCD. This explains the decreased QCT complexation efficacy in the presence of ethanol beyond 0.1 or 0.2 volume fraction.




Similar content being viewed by others
References
D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71.
Zahedi M, Ghiasvand R, Feizi A, Asgari G, Darvish L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double-blind randomized controlled clinical trial. Int J Prev Med. 2013;4:777–85.
Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, Tete S, Madhappan B, Vecchiet J, De Lutiis MA, Caraffa A, Cerulli G. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006;20:47–52.
Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fatty Acids. 1998;58:17–24.
Marsh DT, Das S, Ridell J, Smid SD. Structure-activity relationships for flavone interactions with amyloid β reveal a novel anti-aggregatory and neuroprotective effect of 2’,3’,4’-trihydroxyflavone (2-D08). Bioorg Med Chem. 2017;25:3827–34.
Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–80.
Ohnishi E, Bannai H. Quercetin potentiates TNF-induced antiviral activity. Antivir Res. 1993;22:327–31.
Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7.
Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm. 2011;79:268–75.
Ghasemzadeh A, Jaafar HZ, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15:335–45.
Scambia G, Ranelletti FO, Panici PB, Piantelli M, Bonanno G, De Vincenzo R, Ferrandina G, Rumi C, Larocca LM, Mancuso S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br J Cancer. 1990;62:942–6.
Larocca LM, Piantelli M, Leone G, Sica S, Teofili L, Panici PB, Scambia G, Mancuso S, Capelli A, Ranelletti FO. Type II oestrogen binding sites in acute lymphoid and myeloid leukaemias: growth inhibitory effect of oestrogen and flavonoids. Br J Haematol. 1990;75:489–95.
Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572–82.
Park SN, Lee MH, Kim SJ, Yu ER. Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system. Biochem Biophys Res Commun. 2013;435(3):361–6.
Bose S, Du Y, Takhistov P, Michniak-Kohn B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int J Pharm. 2013;441:56–66.
Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, Zheng Y, Gou M, Huang M, Guo G, Huang N, Qian Z, Wei Y. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4:7021–30.
Liu M, Dong L, Chen A, Zheng Y, Sun D, Wang X, Wang B. Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: spectroscopic study and antioxidant activity. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:854–60.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–57.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25.
Szejtli J. Cylodextrin in drug formulations: part I. Pharm Technol Int. 1991;3:15–23.
Szente L, Szejtli J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv Drug Deliv Rev. 1999;36:17–38.
Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv Drug Deliv Rev. 1999;36:81–99.
D’Aria F, Serri C, Niccoli M, Mayol L, Quagliariello V, Iaffaioli RV, Biondi M, Giancola C. Host–guest inclusion complex of quercetin and hydroxypropyl-β-cyclodextrin. J Therm Anal Calorim. 2017;130:451–6.
Savic IM, Nikolic VD, Savic-Gajic I, Nikolic LB, Radovanovic BC, Mladenovic JD. Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)-β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2015;82:383–94.
Usacheva TR, Sharnin VA. A thermodynamic study of reactions of amino acids with crown ethers in nonaqueous media as examples of guest-host molecular complex formation. Russ Chem Bulletin. 2015;64:2536–44.
Krestov GA, Novosyolov NP. Ionic solvation. New York: Ellis Horwood Edi; 1994.
Donze C, Coleman AW. Solvent effects in competition between guest molecules for β-cyclodextrin. J Incl Phenom Mol Recogn Chem. 1995;23:11–21.
Yoshii H, Kometani T, Furuta T, Watanabe Y, Linko YY, Linko P. Formation of inclusion complexes of cycldextrin with ethanol under anhydrous conditions. Biosci Biotechnol Biochem. 1998;62:2166–70.
Vasiliev VP, Borodin VA, Kozlovsky EV. The use of computers in chemical-analytical calculations, vol. 112. Moscow: Higher Education school; 1993 in Russian.
Borodin VA, Vasil’ev VP, Kozlovskii EV. Mathematical problems of chemical thermodynamics, vol. 219. Novosibirsk: Nauka; 1985 (in Russian).
Usacheva TR, Pham Thi L, Terekhova IV, Kumeev RS, Sharnin VA. Thermodynamics of molecular complexation of glycyl–glycyl–glycine with cryptand [2.2.2] in water–dimethylsulfoxide solvent at 298.15 K. J Therm Anal Calorim. 2016;126:307–14.
Sharnin VA. Thermochemistry of formation of copper (II)-ethylendiamine complexes and solvation of reagents in aqueous organic solvents. J Therm Anal Calorim. 1995;45:721–8.
Zevakin MA, Grazhdan KV, Dushina SV, Sharnin VA. Thermodynamic characteristics of reagents and reaction of Ag+—nicotinamide complex formation in water-ethanol media. J Mol Liq. 2007;131–132:163–7.
Gesse ZF, Repkin GI, Isaeva VA, Sharnin VA. The influence of reagents solvation on enthalpy change of glycine-ion protonation and silver(I) glycine-ion complexation in aqueous-dimethylsulfoxide solutions. J Therm Anal Calorim. 2013;110:1457–62.
Jullian C, Moyano L, Yanez C, Olea-Azar C. Complexation of quercetin with three kinds of cyclodextrins: an antioxidant study. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67:230–4.
Pralhad T, Rajendrakumar K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal. 2004;34:333–9.
Zheng Y, Haworth IS, Zuo Z, Chow MS, Chow AH. Physicochemical and structural characterization of quercetin-β-cyclodextrin complexes. J Pharm Sci. 2005;94:1079–89.
Oprean C, Mioc M, Csányi E, Ambrus R, Bojin F, Tatu C, Cristea M, Ivan A, Danciu C, Dehelean C, Paunescu V, Soica C. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed Pharmacother. 2016;83:1095–104.
Tang P, Tang B, Wang Q, Xu K, Xiong X, Li H. Effect of hydroxypropyl-β-cyclodextrin on the bounding of salazosulfapyridine to human serum albumin. Int J Biol Macromol. 2016;92:105–15.
Hădărugă NG, Hădărugă DI, Isengard HD. “Surface water” and “strong-bonded water” in cyclodextrins: a Karl Fischer titration approach. J Incl Phenom Macrocycl Chem. 2013;75:297–302.
Razmara RS, Daneshfar A, Sahraei R. Solubility of quercetin in water + methanol and water + ethanol from (292.8 to 333.8) K. J Chem. 2010;55:3934–6.
Kanokthip B, Helmut V, Peter W, Luckhana L. Influence of ethanol as a co-solvent in cyclodextrin inclusion complexation: a molecular dynamics study. Sci Pharm. 2015;83:387–99.
Acknowledgements
The calorimetric measurements presented in this work were carried out at the Institute of Thermodynamics and Kinetics of Chemical Processes of the Ivanovo State University of Chemistry and Technology (ISUCT) using the equipment of the Center for Collective Use of ISUCT. The study was carried out under grant of Council on grants of the President of the Russian Federation (Project 14.Z56.18.877-MK). The authors thank the University of Naples Federico II for the financial support of their collaboration contributed to the preparation of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usacheva, T., Kabirov, D., Beregova, D. et al. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J Therm Anal Calorim 138, 417–424 (2019). https://doi.org/10.1007/s10973-019-08136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08136-5