Abstract
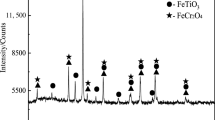
High-chromium vanadium–titanium magnetite (HCVTM) is a good valuable resource with high iron content in the form of complex iron ore which contains various valuable metal elements such as iron, vanadium, titanium, chromium. Direct reduction of HCVTM is studied based on thermodynamic analysis. Combined TG experimental verification and equilibrium calculation model was used to analyze the reaction sequence and equilibrium amount in this paper. The contents in HCVTM reduction system are simplified as 18 kinds of chemical compositions. Reductions of Fe3O4 and FeO·TiO2 are the main reduction reactions and are mainly reduced by C. The reduction reaction sequence of FeO·TiO2 is FeO·TiO2, TiO2, TiC, and Ti; the reduction reaction sequence of Fe3O4 is Fe3O4, FeO, and Fe. The minimum reduction temperature of HCVTM is 860 °C. The reduction of Cr is difficult to implement, and the minimum reduction temperature of V is above 700 °C. The gas phase in this system is mainly CO when the temperature is above 1000 °C. CO partial pressure curve of gasification reaction is in the shape of ‘S’ with increase of temperature. When the temperature is 1350 °C, C/O is 1.0 and reduction time is 30 min, HCVTM can be reduced thoroughly and the reduction degree can reach to 0.98. When C/O is lower than 1.0, FeTi2O5 is the reduction intermediate products from FeO·TiO2. When C/O is 1.0, diffraction peaks of Fe3O4 and FeO·TiO2 disappear, and they are reduced to Fe and TiO2.











Similar content being viewed by others
References
Tang J, Chu MS, Ying ZW, et al. Non-isothermal gas-based direct reduction behavior of high chromium vanadium–titanium magnetite pellets and the melting separation of metallized pellets. Metals. 2017;7(5):153.
Tang J, Chu MS, Feng C, et al. Melting separation behavior and mechanism of high-chromium vanadium-bearing titanomagnetite metallized pellet got from gas-based direct reduction. ISIJ Int. 2016;56(2):210–9.
Hu ML, Liu L, Lv XW, et al. Crystallization behavior of perovskite in the synthesized high-titanium-bearing blast furnace slag using confocal scanning laser microscope. Metall Mater Trans B. 2013;45(1):76–85.
Long HM, Chun TJ, Wang P, et al. Grinding kinetics of vanadium–titanium magnetite concentrate in a damp mill and its properties. Metall Mater Trans B. 2016;47(3):1765–72.
Lv XW, Lun ZG, Yin JQ, et al. Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior. ISIJ Int. 2013;53(7):1115–9.
Chen SY, Fu XJ, Chu MS, et al. Life cycle assessment of the comprehensive utilisation of vanadium titano-magnetite. J Clean Prod. 2015;101:122–8.
Zhang L, Zhang LN, Wang MY, et al. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner Eng. 2007;20(7):684–93.
Zhang GQ, Zhang TA, Zhang Y, et al. Pressure leaching of converter vanadium slag with waste titanium dioxide. Rare Met. 2014;35(7):576–80.
Yuan ZF, Wang XQ, et al. A new process for comprehensive utilization of complex titania ore. Miner Eng. 2006;19(9):975–8.
Chen DS, Song B, Wang LN, et al. Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner Eng. 2011;24(8):864–9.
Samanta S, Mukherjee S, Dey R. Upgrading metals via direct reduction from poly-metallic titaniferous magnetite ore. JOM. 2014;67(2):467–76.
Zhao LS, Wang LN, Chen DS, et al. Behaviors of vanadium and chromium in coal-based direct reduction of high-chromium vanadium-bearing titanomagnetite concentrates followed by magnetic separation. T Nonferr Metal Soc. 2015;25(4):1325–33.
Yu W, Tang QY, Chen JA, et al. Thermodynamic analysis of the carbothermic reduction of a high-phosphorus oolitic iron ore by FactSage. Int J Miner Met Mater. 2016;23(10):1126–32.
He JHO, Kim BS, Park JH. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon. Metall Mater Trans B. 2013;44(6):1352–63.
Xie HQ, Yu QB, Zuo ZL, et al. Thermodynamic analysis of hydrogen production from raw coke oven gas via steam reforming. J Therm Anal Calorim. 2016;126(3):1621–31.
Li P. Thermodynamic analysis of waste heat recovery of molten blast furnace slag. Int J Hydrog Energy. 2017;42(15):9688–95.
Zuo ZL, Yu QB, Wei MQ, et al. Thermogravimetric study of the reduction of copper slag by biomass. J Therm Anal Calorim. 2016;126(2):481–91.
Warczok A, Riveros G. Slag cleaning in crossed electric and magnetic fields. Miner Eng. 2007;20(1):34–43.
Zuo ZL, Yu QB, Xie HQ, et al. Thermodynamic analysis of reduction in copper slag by biomass molding compound based on phase equilibrium calculating model. J Therm Anal Calorim. 2018;132(2):1277–89.
Acknowledgements
The author will acknowledge the support from National Natural Science Foundation of China (Nos. 51604065 & 51674084), the Major State Research Development Program of China (Nos. 2017YFB0603800 & 2017YFB0603804), the Fundamental Funds for the Program of the Science Foundation of Liaoning Province (No. 20170540316), the Fundamental Research Funds for the Central Universities (No. N172507012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, M., Jiang, T., Ding, X. et al. Thermodynamic study of direct reduction of high-chromium vanadium–titanium magnetite (HCVTM) based on phase equilibrium calculation model. J Therm Anal Calorim 136, 885–892 (2019). https://doi.org/10.1007/s10973-018-7687-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7687-8