Abstract
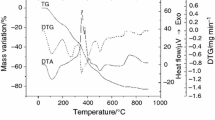
In order to modulate their biological activity in relation with microbial and mammalian cells, new compounds of type M(pmtp)(ClO4)2·nH2O (M: Co, Ni, Cu, Zn; pmtp: 5-phenyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine) were synthesised and characterised by chemical analysis, FAB-MS, IR, UV–Vis–NIR, EPR spectroscopy, cyclic voltammetry and magnetic data at room temperature. The thermal behaviour of these derivatives was also investigated by TG–DSC/MS measurements to evidence the changes induced by heating and also the thermodynamics effects that accompany them. Processes as water elimination, perchlorate decomposition, fragmentation and oxidative degradation of the triazolopyrimidine species were observed during the thermal studies. The in vitro screening of the antimicrobial activity was performed against Gram positive (S. aureus, B. subtilis) and Gram negative (E. coli, K. pneumoniae, P. aeruginosa), both reference and clinical multidrug-resistant bacterial strains and Candida albicans fungal strain. The copper(II) complex was the most active against planktonic microbial cells, exhibiting minimum inhibitory concentration values in the range of 31–125 μg mL−1. Remarkably, all complexes showed an inhibitory activity against biofilm development on the inert substratum, showing a promising potential for the design of new efficient anti-biofilm strategies.







Similar content being viewed by others
References
Ashour HM, Shaaban OG, Rizk OH, El-Ashmawy IM. Synthesis and biological evaluation of thieno [2′, 3′: 4, 5] pyrimido [1, 2-b][1, 2, 4] triazines and thieno [2, 3-d][1, 2, 4] triazolo [1, 5-a] pyrimidines as anti-inflammatory and analgesic agents. Eur J Med Chem. 2013;62:341–51.
Bhatt JD, Chudasama CJ, Patel KD. Pyrazole clubbed triazolo [1, 5-a] pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg Med Chem. 2015;23:7711–6.
Kumar A, Paliwal D, Saini D, Thakur A, Aggarwal S, Kaushik D. A comprehensive review on synthetic approach for antimalarial agents. Eur J Med Chem. 2014;85:147–78.
Wang L, Tian Y, Chen W, Liu H, Zhan P, Li D, Liu H, De Clercq E, Pannecouque C, Liu X. Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 2: discovery of novel [1,2,4]triazolo[1,5-a]pyrimidines using a structure-guided core-refining approach. Eur J Med Chem. 2014;85:293–303.
Sharma A, Kumar V, Khare R, Gupta GK, Beniwal V. Synthesis, docking study, and DNA photocleavage activity of some pyrimidinylhydrazones and 3-(quinolin-3-yl)-5, 7-dimethyl-1, 2, 4-triazolo [4, 3-a] pyrimidine derivatives. Med Chem Res. 2015;24:1830–41.
Łakomska I, Fandzloch M. Application of 1,2,4-triazolo[1,5-a]pyrimidines for the design of coordination compounds with interesting structures and new biological properties. Coord Chem Rev. 2016;327:221–41.
Caballero AB, Marin C, Ramirez-Macias I, Rodriguez-Dieguez A, Quirós M, Salas JM, Sanchez-Moreno M. Structural consequences of the introduction of 2,2′-bipyrimidine as auxiliary ligand in triazolopyrimidine-based transition metal complexes. In vitro antiparasitic activity. Polyhedron. 2012;33:137–44.
Caballero AB, Rodríguez-Diéguez A, Quirós M, Salas JM, Huertas Ó, Ramírez-Macías I, Olmo F, Marín C, Chaves-Lemaur G, Gutierrez-Sánchez R, Sánchez-Moreno M. Triazolopyrimidine compounds containing first-row transition metals and their activity against the neglected infectious Chagas disease and leishmaniasis. Eur J Med Chem. 2014;85:526–34.
Łakomska I, Wojtczak A, Sitkowski J, Kozerski L, Szłyk E. Platinum(IV) complexes with purine anolgs. Studies of molecular structure and proliferative activity in vitro. Polyhedron. 2008;27:2765–70.
Łakomska I, Kooijman H, Spek AL, Shen WZ, Reedijk J. Mono and dinuclear platinum(II) compounds with 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Structure, cytotoxic activity and reaction with 50-GMP. Dalton Trans. 2009;48:10736–41.
Łakomska I, Babinska M, Wojtczak A, Sitkowski J. Synthesis, characterization and in vitro cytotoxicity of three typesof platinum(II) complexes containing 5,7-diethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg Chim Acta. 2016;453:516–21.
Łakomska I, Hoffmann K, Topolski A, Kloskowski T, Drewa T. Spectroscopic, kinetic and cytotoxic in vitro study of hexafluoroglutarate platinum(II) complex with 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg Chim Acta. 2012;387:455–9.
Łakomska I, Fandzloch M, Muzioł T, Sitkowski J, Wietrzyk J. Structure-cytotoxicity relationship for different types of mononuclear platinum(II) complexes with 5,7-ditertbutyl-1,2,4-triazolo[1,5-a]pyrimidine. J Inorg Biochem. 2012;115:100–5.
Hoffmann K, Łakomska I, Wiśniewska J, Kaczmarek-Kędziera A, Wietrzyk J. Acetate platinum(II) compound with 5,7-ditertbutyl-1,2,4-triazolo[1,5-a]pyrimidine that overcomes cisplatin resistance: structural characterization, in vitro cytotoxicity, and kinetic studies. J Coord Chem. 2015;68:3193–208.
Łakomska I, Hoffmann K, Wojtczak A, Sitkowski J, Maj E, Wietrzyk J. Cytotoxic malonate platinum(II) complexes with 1,2,4-triazolo[1,5-a] pyrimidine derivatives: structural characterization and mechanism of the suppression of tumor cell growth. J Inorg Biochem. 2014;141:188–97.
Hoffmann K, Wiśniewska J, Wojtczak A, Sitkowskic J, Denslow A, Wietrzyk J, Jakubowski M, Łakomska I. Rational design of dicarboxylatoplatinum(II) complexes with purine mimetic ligands as novel anticancer agents. J Inorg Biochem. 2017;172:34–45.
Łakomska I, Fandzloch M, Muzioł T, Lis T, Jezierska J. Synthesis, characterization and antitumor properties of two highly cytotoxic ruthenium(III) complexes with bulky triazolopyrimidine ligands. Dalton Trans. 2013;42:6219–26.
Girasolo MA, Attanzio A, Sabatino P, Tesoriere L, Rubino S, Stocco G. Organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine and their cytotoxic activities: the importance of being conformers. Inorg Chim Acta. 2014;423:168–76.
Romero MA, Salas JM, Quirós M. Cobalt(II) complexes of 5,7-dimethyl[1,2,4]-triazolo-[1,5-a]-pyrimidine. Spectroscopic characterization, XRD study and antimicrobial activity. Transit Met Chem. 1993;18:595–8.
Girasolo MA, Schillaci D, Di Salvo C, Barone G, Silvestri A, Ruisi G. Synthesis, spectroscopic characterization and in vitro antimicrobial activity of diorganotin(IV) dichloride adducts with [1,2,4]triazolo-[1,5-a]pyrimidine and 5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidine. J Organomet Chem. 2006;691:693–701.
Girasolo MA, Canfora L, Sabatino P, Schillaci D, Foresti E, Rubino S, Ruisi G, Stocco G. Synthesis, characterization, crystal structures and in vitro antistaphylococcal activity of organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine. J Inorg Biochem. 2012;106:156–63.
Olar R, Calu L, Badea M, Chifiriuc MC, Bleotu C, Velescu B, Stoica O, Ionita G, Stanica N, Silvestro L, Dulea C, Uivarosi V. Thermal behaviour of some biologically active species based on complexes with a triazolopyrimidine pharmacophore. J Therm Anal Calorim. 2017;127:685–96.
Calu L, Badea M, Cerc Korošec R, Bukovec P, Daniliuc C-G, Chifiriuc MC, Măruţescu L, Ciulică C, Serban G, Olar R. Thermal behavior of some novel biologically active complexes with a triazolopyrimidine pharmacophore. J Therm Anal Calorim. 2017;127:697–708.
Măruţescu L, Calu L, Chifiriuc MC, Bleotu C, Daniliuc C-G, Fălcescu D, Kamerzan CM, Badea M, Olar R. Synthesis, physico-chemical characterization, crystal structure and influence on microbial and tumor cells of some Co(II) complexes with 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules. 2017;22:1233–52.
Hathaway BJ. Oxyanions. In: Wilkinson G, Gillard RD, McCleverty JA, editors. Comprehensive coordination chemistry. New York: Pergamon Press; 1987.
Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev. 1971;7:81–122.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, 6th ed., part B. Applications in coordination, organometallic, and bioinorganic chemistry. New Jersey: Wiley; 2009.
Solomon EI, Lever ABP. Inorganic electronic structure and spectroscopy, vol. II, applications and case studies. New York: Wiley; 2006.
The König E, Effect Nephelauxetic. Calculation and accuracy of the interelectronic repulsion parameters I. Cubic high-spin d 2, d 3, d 7 and d 8 systems. Struct Bound. 1972;9:175–372.
Gispert JR. Coordination chemistry. Weinheim: Wiley; 2008.
Hathaway BJ. Copper. In: Wilkinson G, Gillard RD, McCleverty JA, editors. Comprehensive coordination chemistry. New York: Pergamon Press; 1987.
Hathaway BJ, Billing DE. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord Chem Rev. 1970;5:143–207.
Rybak-Akimova EV, Nazarenko AY, Chen L, Krieger PW, Herrera AM, Tarasov VV, Robinson PD. Synthesis, characterization, redox properties, and representative X-ray structures of four- and five-coordinate copper(II) complexes with polydentate aminopyridine ligands. Inorg Chim Acta. 2001;324:1–14.
Yousef TA, Abu El-Reash GM, El-Gammal OA, Bedier RA. Co(II), Cu(II), Cd(II), Fe(III) and U(VI) complexes containing a NSNO donor ligand: synthesis, characterization, optical band gap, in vitro antimicrobial and DNA cleavage studies. J Mol Struct. 2012;1029:149–60.
Zanello P. Inorganic electrochemistry: theory, practice and application. Cambridge: Royal Society of Chemistry; 2003.
Calu L, Badea M, Chifiriuc MC, Bleotu C, David G-I, Ioniţă G, Măruţescu L, Lazăr V, Stanică N, Soponaru I, Marinescu D, Olar R. Synthesis, spectral, thermal, magnetic and biological characterization of Co(II), Ni(II), Cu(II) and Zn(II) complexes with a Schiff base bearing a 1,2,4-triazole pharmacophore. J Therm Anal Calor. 2015;120:375–86.
Olar R, Badea M, Ferbinţeanu M, Stănică N, Alan I. Spectral, magnetic and thermal characterization of new Ni(II), Cu(II), Zn(II) and Cd(II) complexes with a bischelate Schiff base. J Therm Anal Calorim. 2017;127:709–19.
Olar R, Vlaicu ID, Chifiriuc MC, Bleotu C, Stănică N, Vasile Scăeţeanu G, Silvestro L, Dulea C, Badea M. Synthesis, thermal analysis and biological characterisation of some new nickel(II) complexes with unsaturated carboxylates and heterocyclic N-donor ligands. J Therm Anal Calorim. 2017;127:731–41.
Popa M, Hussien MD, Cirstea A, Lazar V, Bezirtzoglou E, Chifiriuc MC, Sakizlian M, Stavropoulou E, Bertesteanu S. Insights on metal based dental implants and their interaction with the surrounding tissues. Curr Top Med Chem. 2015;15:1614–21.
Bertesteanu SVG, Popescu CR, Grigore R, Popescu B. Pharyngoesophageal junction neoplasia-therapeutic management. Chirurgia. 2012;107:33–8.
Acknowledgements
The authors thank for magnetic measurements at room temperature to researcher Nicolae Stanică, from “Ilie Murgulescu” Physical Chemistry Institute of Romanian Academy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badea, M., Calu, L., Korošin, N.Č. et al. Thermal behaviour of some biological active perchlorate complexes with a triazolopyrimidine derivative. J Therm Anal Calorim 134, 665–677 (2018). https://doi.org/10.1007/s10973-018-7134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7134-x