Abstract
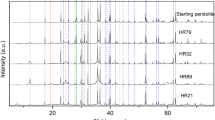
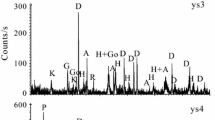
Two varieties of serpentines, antigorite and lizardite originated from dunite and peridotite, respectively, were previously undergone heat treatment in the range from 396 to 1128 °C and then leached via an original approach to the acid processing of dehydrated serpentines. Because of unsaturated (weak) Si–O(Si) bonds existing in the serpentines structure, ortho- [SiO4]4−, di- [Si2O7]6− and other silicate units are easily leached from dehydrated serpentines in the form of soluble silicic acids along with magnesium compounds via this approach, whereas the more complex fragments largely comprised of metasilicate [(SiO3)2−]n chains remain in the unreacted mass The yields of silicic acids produced from heated serpentines are variable. The influence of thermal treatment on both the serpentinite samples was investigated by thermal, X-ray diffraction and chemical analyses. The data derived from the experimental studies allowed to reveal the distribution of primary ortho- [SiO4]4− and meta- [(SiO3)2−]n silicate units in the silicate layers inherent in these serpentine minerals and to understand its influence on the mechanism of their decomposition including the high-temperature phase transformations into forsterite Mg2SiO4 and enstatite Mg2Si2O6.









Similar content being viewed by others
Notes
Mafic rocks have around 50% or less (55–45%) silica and a lot of magnesium and iron, and ultramafic ones contain silica less than 45%, generally more than 18% MgO.
Dunite is an ultramafic plutonic igneous rock. The mineral assemblage is greater than 90% olivine (Mg,Fe)2SiO4 with minor amounts of pyroxene(Mg,Fe)2Si2O6 and other minerals (chromite and pyrope).
Peridotite is an ultramafic plutonic igneous rock consisting mostly of olivine (40–90%) and pyroxene.
Pyroxenite is an ultramafic plutonic igneous rock that consists of dark minerals in the pyroxene group plus a little olivine (10%).
References
Deer W, Howie R, Zussman J. Rock-forming minerals. Sheet silicates, vol. 3. London: Longman; 1962.
le Bas MJ, Streckeisen AL. The IUGS systematics of igneous rocks. J Geol Soc London. 1991;148(5):825–33. doi:10.1144/gsjgs.148.5.0825.
Ball M, Taylor H. The dehydration of chrysotile in air and under hydrothermal conditions. Mineral Mag. 1963;33(261):467–82. doi:10.1180/minmag.1963.033.261.04.
Brindley G, Hayami R. Kinetics and mechanisms of dehydration and recrystallization of serpentine–I. Clays Clay Miner. 1963;12:35–47. doi:10.1346/CCMN.1963.0120107.
Cattaneo A, Gualtieri FA, Artioli G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys Chem Miner. 2003;30(3):177–83. doi:10.1007/s00269-003-0298-2.
Zaremba T, Krząkała A, Piotrowski J, Garczorz D. Study on the thermal decomposition of chrysotile asbestos. J Therm Anal Calorim. 2010;101(2):479–85. doi:10.1007/s10973-010-0819-4.
Kusiorowski R, Zaremba T, Piotrowski J, Adamek J. Thermal decomposition of different types of asbestos. J Therm Anal Calorim. 2012;109(2):693–704. doi:10.1007/s10973-012-2222-9.
Hršak D, Sučik G, Lazić L. The thermophysical properties of serpentinite. Metalurgija. 2008;47(1):29–31.
Viti C. Serpentine minerals discrimination by thermal analysis. Am Mineral. 2010;95(4):631–8. doi:10.2138/am.2010.3366.
Bloise A, Catalano M, Barrese E, Gualtieri AF, Bursi Gandolfi N, Capella S, et al. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J Therm Anal Calorim. 2016;123(3):2225–39. doi:10.1007/s10973-015-4939-8.
MacKenzie KJD, Meinhold RH. Thermal reactions of chrysotile revisited; a 29 Si and 25 Mg MAS NMR study. Am Mineral. 1994;79(1–2):43–50.
Gürtekin G, Albayrak M. Thermal reaction of antigorite: a XRD, DTA-TG work. Bull Miner Res Explor. 2006;133:41–9.
Trittschack R, Grobéty B. Dehydroxylation kinetics of lizardite. Eur J Mineral. 2012;24(1):47–57. doi:10.1127/0935-1221/2012/0024-2169.
Trittschack R, Grobéty B, Brodard P. Kinetics of the chrysotile and brucite dehydroxylation reaction: a combined non-isothermal/isothermal thermogravimetric analysis and high-temperature X-ray powder diffraction study. Phys Chem Miner. 2013;41(3):197–214. doi:10.1007/s00269-013-0638-9.
Alizadehhesari K, Golding SD, Bhatia SK. Kinetics of the dehydroxylation of serpentine. Energy Fuels. 2012;26(2):783–90. doi:10.1021/ef201360b.
Gualtieri AF, Giacobbe C, Viti C. The dehydroxylation of serpentine group minerals. Am Mineral. 2012;97(4):666. doi:10.2138/am.2012.3952.
Zulumyan NO, Isaakyan AR, Oganesyan ZG. A new promising method for processing of serpentinites. Russ J Appl Chem. 2007;80(6):1020–2. doi:10.1134/S1070427207060353.
Zulumyan NH, Papakhchyan LR, Isahakyan AR, Beglaryan HA, Terzyan AM. Study of mechanical impact on the crystal lattice of serpentines. Geochem Int. 2011;49(9):937–41. doi:10.1134/S0016702911090084.
Zulumyan NH, Papakhchyan LR, Terzyan AM, Beglaryan HA, Isahakyan AR. Structural features of the silicate networks of serpentines. Theor Found Chem Eng. 2013;47(2):185–90. doi:10.1134/S0040579512040215.
Zulumyan N, Mirgorodski A, Isahakyan A, Beglaryan H. The mechanism of decomposition of serpentines from peridotites on heating. J Therm Anal Calorim. 2014;115(2):1003–12. doi:10.1007/s10973-013-3483-7.
Zulumyan NH, Papakhchyan LR, Isahakyan AR, Beglaryan HA, Aloyan SG. Effect of mechanical treatment on the silicate lattice of kaolinite. Russ J Phys Chem A. 2012;86(12):1887–91. doi:10.1134/S003602441212028X.
Zulumyan NH, Papakhchyan LR, Isahakyan AR, Beglaryan HA, Aloyan SG. The influence of mechanical treatment on the silicate network of talc. Russ J Phys Chem A. 2012;86(6):1008–13. doi:10.1134/S0036024412060350.
Velinskii VV, Gusev GM. Production of extra pure silica from serpentinites. J Min Sci. 2002;38(4):402–4. doi:10.1023/a:1023324206554.
Teir S, Revitzer H, Eloneva S, Fogelholm C-J, Zevenhoven R. Dissolution of natural serpentinite in mineral and organic acids. Int J Miner Process. 2007;83(1–2):36–46. doi:10.1016/j.minpro.2007.04.001.
Gladikova LA, Teterin VV, Freidlina RG. Production of magnesium oxide from solutions formed by acid processing of serpentinite. Russ J Appl Chem. 2008;81(5):889–91. doi:10.1134/s1070427208050339.
Fedoročková A, Hreus M, Raschman P, Sučik G. Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner Eng. 2012;32:1–4. doi:10.1016/j.mineng.2012.03.006.
Sučik G, Szabóová A, Popovič L, Hršak D. The relationship between thermal treatment of serpentine and its reactivity. Mater Technol. 2016;50(1):55–8. doi:10.17222/mit.2014.222.
Zulumyan NO, Isaakyan AR, Pirumyan PA, Beglaryan AA. The structural characteristics of amorphous silicas. Russ J Phys Chem A. 2010;84(4):791–3. doi:10.1134/S003602441004031X.
Isahakyan AR, Beglaryan HA, Pirumyan PA, Papakhchyan LR, Zulumyan NH. An IR spectroscopic study of amorphous silicas. Russ J Phys Chem A. 2011;85(1):72–5. doi:10.1134/S0036024410121015.
Torosyan A, Zulumyan N, Hovhannisyan Z, Ghazaryan S, editors. The influence of mechanical processing on the process of thermal reduction of SiO 2 by Al powder. In: EPD Congress 2005 as held at the 2005 TMS Annual Meeting; 2005.
Zulumyan N, Isahakyan A, Hovhannisyan Z, Torosyan A. The influence of mechanical activation on the process of thermal reduction of silica by magnesium powder. Magnes Technol. 2006;2006:351–4.
Zulumyan N, Mirgorodski A, Isahakyan A, Beglaryan H, Gabrielyan A, Terzyan A. A low-temperature method of the β—wollastonite synthesis. J Therm Anal Calorim. 2015;122(1):97–104. doi:10.1007/s10973-015-4752-4.
Kirschenbaum H. The classical chemical analysis of silicate rocks: the old and the new, vol. 1547. Washington: Geological survey bulletin; 1983.
Langford JI, Wilson AJC. Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Crystallogr. 1978;11(2):102–13. doi:10.1107/S0021889878012844.
Acknowledgements
This work was supported by the RA MES State Committee of Science, in the frames of the research project no 16YR-1D025. Authors are very grateful to Dr. M. Mellini for providing the CHR-23 sample and information about its occurrence.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulumyan, N., Isahakyan, A., Beglaryan, H. et al. A study of thermal decomposition of antigorite from dunite and lizardite from peridotite. J Therm Anal Calorim 131, 1201–1211 (2018). https://doi.org/10.1007/s10973-017-6705-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6705-6