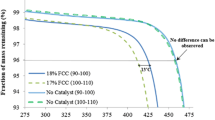
Abstract
Polymer degradation by the catalytic cracking process has been investigated as an important way of hydrocarbon recovery with high added value. In addition, the effects of the catalyst properties and their behavior during the catalytic degradation of polyolefins have been studied using thermogravimetric analysis. In this context, the present work compares the interactions between the polymeric molecules and the contact surface of zeolitic catalysts (hierarchical and standard Beta zeolites, Y zeolite, ZSM-5, ZSM-12 and MCM-22) with different properties, in the catalytic cracking reactions of low-density polyethylene. It was also possible to evaluate distinct reaction mechanisms in function of the thermal homogenization procedure employed by thermogravimetric analysis. Among the zeolites with large pore diameters, the hierarchical Beta showed superior external area due to the use of a bulky silanized agent (phenylaminopropyl-trimethoxysilane) in the synthesis. In cracking reactions, the polymeric macromolecules mainly react in the external surface of the catalysts due to the several diffusional limitations imposed. Thus, the zeolitic catalysts exhibited behaviors respective to a combination of accessibility to the active centers and selectivity in function of the acid strength of the centers. So, based on this process, zeolites with larger external area values (hierarchical Beta, standard Beta, ZSM-5 and MCM-22) promoted the lower values of degradation temperature with high efficiency of conversion.
Graphical Abstract







Similar content being viewed by others
References
Ali MF, Ahmed S, Qureshi MS. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels. Fuel Process Technol. 2011;92:1109–20.
Moinuddin SM, Mohammad MR, Mohammed M. Abundant high-density polyethylene (HDPE-2) turns into fuel by using of HZSM-5 catalyst. J Fundam Renew Energy Appl. 2011;1:1–12.
Rapacz-Kmita A, Gajek M, Dudek M, Stodolak-Zych E, Szaraniec B, Lach R. Thermal, structural and mechanical analysis of polymer/clay nanocomposites with controlled degradation. J Therm Anal Calorim. 2017;127:389–98.
Valanciene E, Miknius L, Pedisius N. The influence of zeolite catalyst on kinetics and thermodynamics of polypropylene waste thermal degradation. J Therm Anal Calorim. 2016;124:341–54.
Elordi G, Olazar M, Lopez G, Amutio M, Artetxe M, Aguado R, Bilbao J. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor. Anal Appl Pyrolysis. 2009;85:345–51.
Aguado J, Serrano DP, Miguel GS, Escola JM. Catalytic activity of zeolitic and mesostructured catalysts in the cracking of pure and polyolefins. J Anal Appl Pyrolysis. 2007;78:153–61.
Al-Salem SM, Lettieri P, Baeyens J. Recycling and recovery routes of plastic solid waste (PSW). Rev Waste Manag. 2009;29:2625–43.
Xu J, Jung K, Atme A, Shanmugam S, Boyer C. A robust and versatile photoinduced living polymerization of conjugated and unconjugated monomers and its oxygen tolerance. J Am Chem Soc. 2014;136:5508–19.
Vidal F, Gowda RR, Chen EYX. Chemoselective, stereospecific, and living polymerization of polar divinyl monomers by chiral zirconocenium catalysts. J Am Chem Soc. 2015;137:9469–80.
Hujuri U, Ghoshal AK, Gumma S. Modeling pyrolysis kinetics of plastic mixtures. Polym Degrad Stab. 2008;93:1832–7.
Liu WJ, Tian K, Jiang H, Zhang XS, Yang GX. Preparation of liquid chemical feedstocks by co-pyrolysis of electronic waste and biomass without formation of polybrominated dibenzo-p-dioxins. Bioresour Technol. 2013;128:1–7.
Liu W, Hu C, Yang Y, Tong D, Li G, Zhu L. Influence of ZSM-5 zeolite on the pyrolytic intermediates from the co-pyrolysis of pubescens and LDPE. Energy Convers Manag. 2010;51:1025–32.
Ma C, Yu J, Wang B, Song Z, Xiang J, Hu S, Su S, Sun L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: a review. Renew Sustain Energy Rev. 2016;61:433–50.
Soják L, Kubinec R, Jurdáková H, Bajus M. High resolution gas chromatographic–mass spectrometric analysis of polyethylene and polypropylene thermal cracking products. J Anal Appl Pyrolysis. 2007;78:387–99.
Serrano DP, Aguado J, Escola JM. Developing advanced catalysts for the conversion of polyolefinic waste plastics into fuels and chemicals. ACS Catal. 2012;2:1924–41.
Juárez-Hernández L, Pérez-Pariente J, Aguilar-Pliego J, Múgica-Álvarez V, Gutiérrez-Arzaluz M, Grande MS, Márquez-Álvarez C, Sastre E. Mesoporous materials with enhanced porosity and acidity to obtain clean fuels from low-density polyethylene (LDPE) cracking. J Porous Mater. 2015;22:269–81.
Roy GK, Bipin K, Jha S. Chromatographic study of the recovered gases from hydropyrolytic de-polymerization of LDPE, MDPE and HDPE mix type of waste polyethylene. Appl Petrochem Res. 2016;6:65–72.
Durmuş A, Naci Koç S, Selda Pozan G, Kaşgöz A. Thermal-catalytic degradation kinetics of polypropylene over BEA, ZSM-5 and MOR zeolites. Appl Catal B Environ. 2005;61:316–22.
Marcilla A, Gómez-Siurana A, Valdés F. Catalytic cracking of low-density polyethylene over H-Beta and HZSM-5 zeolites: Influence of the external surface. Kinetic model. Polym Degrad Stab. 2007;92:197–204.
Boughattas I, Ferry M, Dauvois V, Lamouroux C, Dannoux-Papin A, Leoni E, Balanzat E, Esnouf S. Thermal degradation of γ-irradiated PVC: I-dynamical experiments. Polym Degrad Stab. 2016;26:219–26.
Marcilla A, Beltrán MI, Navarro R. TG/FT-IR analysis of HZSM5 and HUSY deactivation during the catalytic pyrolysis of polyethylene. J Anal Appl Pyrolysis. 2006;76:222–9.
Garforth A, Fiddy S, Lin YH, Ghanbari-Siakhali A, Sharratt PN, Dwyer J. Catalytic degradation of high density polyethylene: an evaluation of mesoporous and microporous catalysts using thermal analysis. Thermochim Acta. 1997;294:65–9.
Coelho A, Costa L, Marques MM, Fonseca IM, Lemos MANDA, Lemos F. The effect of ZSM-5 zeolite acidity on the catalytic degradation of high-density polyethylene using simultaneous DSC/TG analysis. Appl Catal A Gen. 2012;413–414:183–91.
Gobin K, Manos G. Thermogravimetric study of polymer catalytic degradation over microporous materials. Polym Degrad Stab. 2004;86:25–31.
Park JW, Kim J, Seo G. The effect of pore shape on the catalytic performance of zeolites in the liquid-phase degradation of HDPE. Polym Degrad Stab. 2002;76:495–501.
Li K, Valla J, Garcia-Martinez J. Realizing the commercial potential of hierarchical zeolites: new opportunities in catalytic cracking. ChemCatChem. 2014;6:46–66.
Möller K, Bein T. Mesoporosity-a new dimension for zeolites. Chem Soc Rev. 2013;2013(42):3689–707.
Perez-Ramirez J, Christensen CH, Egeblad K, Christensen CH, Groen JC. Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem Soc Rev. 2008;37:2530–42.
Čejka J, Centi G, Perez-Pariente J, Roth WJ. Zeolite-based materials for novel catalytic applications: opportunities, perspectives and open problems. Catal Today. 2012;179:2–15.
Serrano DP, Escola JM, Pizarro P. Synthesis strategies in the search for hierarchical zeolites. Chem Soc Rev. 2013;42:4004–35.
Serrano DP, Pinnavaia TJ, Aguado J, Escola JM, Peral A, Villalba L. Hierarchical ZSM-5 zeolites synthesized by silanization of protozeolitic units: mediating the mesoporosity contribution by changing the organosilane type. Catal Today. 2014;227:15–25.
Milina M, Mitchell S, Michels NL, Kenvin J, Perez-Ramirez J. Interdependence between porosity, acidity, and catalytic performance in hierarchical ZSM-5 zeolites prepared by post-synthetic modification. J Catal. 2013;308:398–407.
Ishihara A, Inui K, Hashimoto T, Nasu H. Preparation of hierarchical b and Y zeolite-containing mesoporous sílica-aluminas and their properties for catalytic cracking of n-dodecane. J Catal. 2012;295:81–90.
Ramasamya KK, Zhang H, Sunb J, Wanga Y. Conversion of ethanol to hydrocarbons on hierarchical HZSM-5 zeolites. Catal Today. 2014;238:103–10.
Bleken FL, Barbera K, Bonino F, Olsbye U, Lillerud KP, Bordiga S, Beato P, Janssens TVW, Svelle S. Catalyst deactivation by coke formation in microporous and desilicated zeolite H-ZSM-5 during the conversion of methanol to hydrocarbons. J Catal. 2013;307:62–73.
Svelle S, Sommer L, Barbera K, Vennestrøm PNR, Olsbye U, Lillerud KP, Bordiga S, Pan YH, Beato P. How defects and crystal morphology control the effects of desilication. Catal Today. 2011;168:38–47.
Serrano DP, Escola JM, Briones L, Medina S, Martinez A. Hydroreforming of the oils from LDPE thermal cracking over Ni–Ru and Ru supported over hierarchical Beta zeolite. Fuel. 2015;144:287–94.
Aguado J, Serrano DP, Escola JM, Briones L. Deactivation and regeneration of a Ni supported hierarchical Beta zeolite catalyst used in the hydroreforming of the oil produced by LDPE thermal cracking. Fuel. 2013;109:679–86.
García-Muñoz RA, Serrano DP, Vicente G, Linares M, Vitvarova D, Cejka J. Remarkable catalytic properties of hierarchical zeolite-Beta in epoxide rearrangement reactions. Catal Today. 2015;243:141–52.
Pérez-ramírez J, Abelló S, Bonilla A, Groen JC. Tailored mesoporosity development in zeolite crystals by partial detemplation and desilication. Adv Funct Mater. 2009;19:164–72.
Caldeira VPS, Peral A, Linares M, Araujo AS, Garcia-Muñoz RA, Serrano DP. Properties of hierarchical Beta zeolites prepared from protozeolitic nanounits for the catalytic cracking of high density polyethylene. Appl Catal A Gen. 2016;. doi:10.1016/j.apcata.2016.11.003.
Camblor MA, Corma A, Mifsud A, Pérez-Pariente J, Valencia S. Synthesis of nanocrystalline zeolite beta in the absence of alkali metal cations. Stud Surf Sci Catal. 1997;105:341–8.
Argauer RJ, Landolt GR (1978) US Pat. RE 29857.
Caldeira VPS, Santos AGD, Pergher SBC, Costa MJF, Araujo AS. Use of a low-cost template-free ZSM-5 for atmospheric petroleum residue pyrolysis. Quim Nova. 2016;39–3:292–7.
Araujo AS, Silva AOS, Souza MJB, Coutinho ACSLS, Aquino JMFB, Moura JA, Pedrosa AMG. Crystallization of ZSM-12 zeolite with different Si/Al ratio. Adsorption. 2005;11:159–65.
Pergher SBC, Corma A, Fornés V. Preparation and characterization of MCM-22 zeolite and its layered precursor. Quim Nova. 2003;26:795–802.
Calgaroto C, Scherer RP, Calgaroto S, Oliveira JV, Oliveira D, Pergher SBC. Immobilization of porcine pancreatic lipase in zeolite MCM 22 with different Si/Al ratios. Appl Catal A Gen. 2011;394:101–4.
Database of Zeolite Structures. International Zeolite Association 2017. http://www.iza-strucuture.org. Accessed 03 Mar 2017.
Morales-Pacheco P, Alvarez F, Bucio L, Domínguez JMJ, Phys J. Synthesis and structural properties of zeolitic nanocrystals II: FAU-type zeolites. Chem C. 2009;113:2247–55.
Holmberg BA, Wang H, Yan Y. High silica zeolite Y nanocrystals by dealumination and direct synthesis. Microporous Mesoporous Mater. 2004;74:189–98.
Aguado J, Serrano DP, Rodríguez JM. Zeolite Beta with hierarchical porosity prepared from organofunctionalized seeds. Microporous Mesoporous Mater. 2008;115:504–13.
Kore R, Srivastava R, Satpati B. Synthesis of industrially important aromatic and heterocyclic ketones using hierarchical ZSM-5 and Beta zeolites. Appl Catal A Gen. 2015;493:129–41.
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;. doi:10.1515/pac-2014-1117.
Lin X, Zhang Z, Sun J, Guo W, Wang Q. Effects of phosphorus-modified HZSM-5 on distribution of hydrocarbon compounds from wood–plastic composite pyrolysis using Py-GC/MS. J Anal Appl Pyrolysis. 2015;116:223–30.
Acknowledgements
The authors are immensely grateful to the Department of Chemical and Environmental Technology of the Rey Juan Carlos University (URJC, Spain) for performing the ammonia TPD analyses. The authors also thank the Foundation for the Coordination and Improvement of Higher Level or Education Personnel (CAPES, Brazil) for its financial support to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caldeira, V.P.S., Santos, A.G.D., Oliveira, D.S. et al. Polyethylene catalytic cracking by thermogravimetric analysis. J Therm Anal Calorim 130, 1939–1951 (2017). https://doi.org/10.1007/s10973-017-6551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6551-6