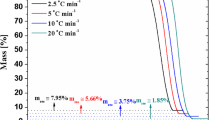
Abstract
Kinetic analysis of the non-isothermal degradation of poly(ethylene oxide) has been performed using isoconversional model-free methods, model-fitting methods, invariant kinetic parameters method and Netzsch Thermokinetics software in order to establish whether different kinetic approaches yield consistent kinetic parameters. It has been shown that these approaches yield consistent kinetic parameters and can be combined in such a way as to enhance the reliability and quality of each other and consequently the overall kinetic analysis. The most probable kinetic parameters for the non-isothermal degradation of poly(ethylene oxide) have been determined. These kinetic parameters have been used for prediction of isothermal kinetics of poly(ethylene oxide), and their potential for reliable prediction has been noticed.






Similar content being viewed by others
References
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic of thermally stimulated processes. Cham: Springer; 2015. p. 9–10.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand. 1966;70A:487–523.
Ozawa T. A new method of analysing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–9.
Friedman HL. Kinetic of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic resin. J Polym Sci Part C. 1963;6:183–95.
Kissinger HE. Reaction kinetic in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Vyazovkin S. Advanced isoconversional method. J Therm Anal. 1997;49:1991–9.
Li CR, Tang TB. A new method for analysing non-isothermal thermoanalytical data from solid-state reactions. Thermochim Acta. 1999;325:43–6.
Vyazovkin S. A unified approach to kinetic processing in nonisothermal data. Int J Chem Kinet. 1996;28:95–101.
Málek J. The kinetic analysis of nonisothermal data. Thermochim Acta. 1992;200:257–69.
Criado JM, Málek J, Ortega A. Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim Acta. 1989;147:377–85.
Lesnikovich AI, Levchik SV. A method of finding invariant values of kinetic parameters. J Therm Anal. 1983;27:89–93.
Chrissafis K. Kinetic of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li CR, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of kinetic analysis: part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2001;115:1780–91.
Rotaru A, Gosa M, Rotaru P. Computational thermal and kinetic analysis. Software for non-isothermal kinetics by standard procedure. J Therm Anal Calorim. 2008;94:367–71.
Rotaru A, Gosa M, Rotaru P. Computational thermal and kinetic analysis. Complete standard procedure to evaluate the kinetic triplet from non-isothermal data. J Therm Anal Calorim. 2009;97:421–6.
Stipanelov Vrandečić N, Erceg M, Jakić M, Klarić I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim Acta. 2010;498:71–80.
Pielichowski K, Flejtuch K. Non-oxidative thermal degradation of poly(ethylene oxide): kinetic and thermoanalytical study. J Anal Appl Pyrolysis. 2005;73:131–8.
Manoratne CH, Rajapakse RMG, Dissanayake MAKL. Ionic conductivity of poly(ethylene oxide) (PEO)-montmorillonite nanocomposites prepared by intercalation from aqueous medium. Int J Electrochem Sci. 2006;1:32–46.
Liao C-S, Ye W-B. Enhanced ionic conductivity in poly(ethylene oxide)/layered double hydroxide nanocomposites electrolytes. J Polym Res. 2003;10:241–6.
Chen H-W, Chang F-C. The novel polymer electrolyte nanocomposite composed of poly(ethylene oxide), lithium triflate and mineral clay. Polymer. 2001;42:9763–9.
Quartatone E, Mustarelli P, Magistris A. PEO-based composite polymer electrolytes. Solid State Ion. 1998;110:1–14.
Jhang WC, Chen WC, Wang YW, Chang RH, Shu CM. Thermal stability evaluation of lithium-ion polymer batteries. J Therm Anal Calorim. 2015;122:1099–105.
Pielichowska K, Głowinkowskib S, Lekkic J, Biniaśd D, Pielichowskia K, Jenczykb J. PEO/fatty acid blends for thermal energy storage materials. Structural/morphological features and hydrogen interactions. Eur Polym J. 2008;44:3344–60.
Fu X, Zhang Y, Kong W, Chen X, Wang J, Zhou C, Lei J. Synthesis and properties of bulk-biodegradable phase change materials based on polyethylene glycol for thermal energy storage. J Therm Anal Calorim. 2016. doi:10.1007/s10973-016-5959-8z.
Budrugeac P, Segal E. Some methodological problems concerning nonisothermal kinetic analysis of heterogeneous solid–gas reactions. Int J Chem Kinet. 2001;33:564–73.
Pérez-Maqueda LA, Criado LM, Gotor FJ, Malek J. Advantages of combined kinetic analysis of experimental data obtained under any heating profile. J Phys Chem A. 2002;106:2862–8.
Netzsch Thermokinetics Software Manual, Selb: Netzsch-Gerätebau GmbH; 2014.
Opfermann J. Kinetic analysis using multivariate non-linear regression: I. Basic concepts. J Therm Anal Calorim. 2000;60:641–58.
Madorsky SL, Strauss S. Thermal degradation of polyethylene oxide and polypropylene oxide. J Polym Sci. 1959;36:183–94.
Audebert R, Aubineau C. Etude par thermogravimetrie dynamique de la degradation thermique des polymeres. Eur Polym J. 1970;6:965–79.
Calahorra E, Cortezar M, Guzman GM. Thermal decomposition of poly(ethylene oxide), poly(methyl methacrylate) and their mixtures by thermogravimetric method. J Polym Sci B. 1985;23:257–60.
Wang FY, Ma CCM, Wu WJ. Kinetic parameters of thermal degradation of polyethylene glycol-toughened novolac-type phenolic resin. J Appl Polym Sci. 2001;80:188–96.
Barbadillo F, Mier JL, Artiaga R, Losada R, Garcia L. In: Proceedings of the 8th European symposium on thermal analysis and calorimetry: ESTAC 8, August 25–29, 2002, Barcelona, Spain. Budapest: Akadémiai Kiadó; 2002. p. 75.
Erceg M, Krešić I, Jakić M, Andričić B. Kinetic analysis of poly(ethylene oxide)/lithium montmorillonite nanocomposites. J Therm Anal Calorim. 2016. doi:10.1007/s10973-016-5413-y.
Jakić M, Stipanelov Vrandečić N, Erceg M. Kinetic analysis of the non-isothermal degradation of poly(vinyl chloride)/poly(ethylene oxide) blends. J Therm Anal Calorim. 2016;123:1513–22.
Jakić M, Stipanelov Vrandečić N, Erceg M. Thermal degradation of poly(3-hydroxybutyrate)/poly(ethylene oxide) blends: thermogravimetric and kinetic analysis. Eur Polym J. 2016;81:376–85.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejon A, Criado JM. Limitations of model-fitting methods for kinetic analysis: polystyrene thermal degradation. Resour Conserv Recyl. 2013;74:75–81.
Pérez-Maqueda LA, Criado JM, Sánchez-Jiménez PE. Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A. 2006;110:12456–62.
Rotaru A. Discriminating within the kinetic models for heterogeneous processes of materials by employing a combined procedure under TKS-SP 2.0 software. J Therm Anal Calorim. 2016;126(2):919–32.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erceg, M., Krešić, I., Vrandečić, N.S. et al. Different approaches to the kinetic analysis of thermal degradation of poly(ethylene oxide). J Therm Anal Calorim 131, 325–334 (2018). https://doi.org/10.1007/s10973-017-6349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6349-6