Abstract
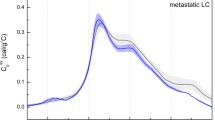
Preoperative neoadjuvant chemotherapy is currently a common approach for treatment of breast cancer patients. These anticancer drugs are harmful, but essential in the fight against tumor cells. The purpose of this pilot study was to compare the thermal changes of blood plasma with differential scanning calorimetry (DSC) method between neoadjuvant chemotherapy received and not received breast cancer patients. The study included 11 adult breast cancer women who were grouped according to TNM stage and perioperative treatments. Patients who had ductal carcinoma in situ (DCIS), as a local tumor without any metastases did not receive any neoadjuvant chemotherapy (n = 3). Patients with invasive lobular carcinoma with local metastases received neoadjuvant chemotherapy preoperatively (n = 8). Peripheral blood samples were collected from the patients before the operation and from healthy female controls (n = 5) ranging from 26 to 60 years old. Denaturation of plasma components was detected in SETARAM Micro DSC-II calorimeter. Our results showed in control group two main T ms (~56 and 63 °C as averages) with ΔH ~ 1.16 J g−1. In DCIS samples three T ms (~55.5; 60.6 and 65.5 °C) with ΔH ~ 1.01 J g−1, while in group with neoadjuvant chemotherapy four T ms (56.3; 60.9; 66.3 and 74.4 °C) with ΔH = 1.07 J g−1 were observed in average. These measurements demonstrated that neoadjuvant chemotherapy aggravates the thermodynamic changes in the blood plasma samples of invasive breast cancer patients.

Similar content being viewed by others
References
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:8–30.
Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg. 2013;148:971–9.
Wuttke M, Phillips KA. Clinical management of women at high risk of breast cancer. Curr Opin Obstet Gynecol. 2015;27:6–13.
Masarwah A, Auvinen P, Sudah M, Rautiainen S, Sutela A, Pelkonen O, Oikari S, Kosma V-M, Vanninen R. Very low mammographic breast density predicts poorer outcome in patients with invasive breast cancer. Eur Radiol. 2015;25:1875–82.
Van Cleef A, Altintas S, Huizing M, Papadimitriou K, Van Dam P, Tjalma W. Current view on ductal carcinoma in situ and importance of the margin thresholds: a review. Facts Views Vis Obgyn. 2014;6:210–8.
Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12:115–24.
Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23:231–6.
Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67.
Garbett NC, Miller JJ, Jenson AB, Chaires JB. Calorimetric analysis of the plasma proteome. Semin Nephrol. 2007;27:621–6.
Fekecs T, Zapf I, Ferencz A, Lőrinczy D. Differential scanning calorimetry (DSC) analysis of human plasma in melanoma patients with or without regional lymph node metastases. J Therm Anal Calorim. 2012;108:149–52.
Todinova S, Krumova S, Gartcheva L, Robeerst C, Taneva SG. Microcalorimetry of blood serum proteome: a modified interaction network in the multiple myeloma case. Anal Chem. 2011;83:7992–8.
Chagovetz AA, Quinn C, Damarse N, Hansen LD, Chagovetz AM, Jensen RL. Differential scanning calorimetry of gliomas: a new tool in brain cancer diagnostics? Neurosurgery. 2013;73:289–95.
Garbett NC, Mekmaysy CS, DeLeeuw L, Chaires JB. Clinical application of plasma thermograms: utility, practical approaches and considerations. Methods. 2015;76:41–50.
Vega S, Garcia-Gonzalez MA, Lanas A, Velazquez-Campoy A, Abian A. Deconvolution analysis for classifying gastric adenocarcinoma patients based on differential scanning calorimetry serum thermograms. Sci Rep. 2015;5:7988.
Todinova S, Krumova S, Kurtev P, Dimitrov V, Djongov L, Dudunkov Z, Taneva SG. Calorimetry-based profiling of blood plasma from colorectal cancer patients. Biochim Biophys Acta. 2012;1820:1879–85.
Fish DJ, Brewood GP, Kim JS, Garbett NC, Chaires JB, Benight AS. Statistical analysis of plasma thermograms measured by differential scanning calorimetry. Biophys Chem. 2010;152:184–90.
Garbett NC, Merchant ML, Helm CW, Jenson AB, Klein JB, Chaires JB. Detection of cervical cancer biomarker patterns in blood plasma and urine by differential scanning calorimetry and mass spectrometry. PLoS One. 2014;9:e84710.
Zapf I, Fekecs T, Ferencz A, Tizedes GY, Pavlovics G, Kalmán E, Lőrinczy D. DSC analysis of human plasma in breast cancer patients. Thermochim Acta. 2011;524:88–91.
Zapf I, Moezzi M, Fekecs T, Nedvig K, Lőrinczy D, Ferencz A. Influence of oxidative injury and monitoring of blood plasma by DSC on breast cancer patients. J Therm Anal Calorim. 2015. doi:10.1007/s10973-015-4642-9.
Kikalishvili L, Ramishvili M, Nemsadze G, Lezhava T, Khorava P, Gorgoshidze M, Kiladze M, Monaselidze J. Thermal stability of blood plasma proteins of breast cancer patients, DSC study. J Therm Anal Calorim. 2015;120:501–5.
Fatima T, Roohi N, Abid R. Circulatory proteins in women with breast cancer and their chemotherapeutic responses. Pak J Zool. 2013;45:1207–13.
Moezzi M, Ferencz A, Lőrinczy D. Evaluation of blood plasma changes by differential scanning calorimetry in psoriatic patients treated with drugs. J Therm Anal Calorim. 2014;116:557–62.
Könczöl F, Wiegand N, Nőt LG, Lőrinczy D. Examination of the cyclophosphamide induced polyneuropathy on guinea pig sciatic nerve and gastrocnemius muscle with differential scanning calorimetry. J Therm Anal Calorim. 2014;115:2239–43.
Michnik A, Polaczek-Grelik K, Leśniak P, Drzazga Z. Effects of low-dose ionizing radiation on α, β-globulins solutions studied by DSC. J Therm Anal Calorim. 2013;111:1845–52.
Michnik A, Drzazga Z. Thermal denaturation of mixtures of human serum proteins, DSC study. J Therm Anal Calorim. 2010;101:513–8.
Chu HL, Chen TH, Wu CY, Yang YC, Tseng SH, Cheng TM, Ho LP, Tsai LY, Li HY, Chang CS, Chang CC. Thermal stability and folding kinetics analysis of disordered protein, securin. J Therm Anal Calorim. 2014;115:2171–8.
Moezzi M, Zapf I, Fekecs T, Nedvig K, Lőrinczy D, Ferencz A. Influence of oxidative injury and monitoring of blood plasma by DSC on patients with psoriasis. J Therm Anal Calorim. 2015. doi:10.1007/s10973-015-4674-1.
Splinter R, van Herwaarden AW, van Wetten IA, Pfreundt A, Svendsen WE. Fast differential scanning calorimetry of liquid samples with chips. Thermochim Acta. 2014;603:162–71.
Arias-Moreno X, Abian O, Vega S, Sancho J, Velazquez-Campoy A. Protein–cation interactions: structural and thermodynamic aspects. Curr Protein Pept Sci. 2011;12:325–38.
Garbett NC, Chaires JB. Thermodynamic studies for drug design and screening. Expert Opin Drug Discov. 2012;7:299–314.
Acknowledgements
This work was supported by Grants OTKA CO-272 (for Dénes Lőrinczy). The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ferencz, A., Zapf, I. & Lőrinczy, D. Harmful effect of neoadjuvant chemotherapy monitoring by DSC on breast cancer patients’ blood plasma. J Therm Anal Calorim 126, 55–59 (2016). https://doi.org/10.1007/s10973-016-5291-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5291-3