Abstract
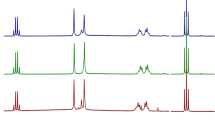
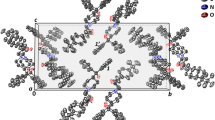
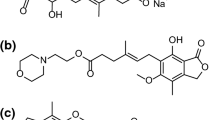
DA-6886 is a new 5-HT4 receptor agonist under development for the treatment of constipation-predominant irritable bowel syndrome. The objective of this work was to investigate the existence of polymorphs and pseudopolymorphs of DA-6886. Five crystal forms of DA-6886 have been isolated by recrystallization and characterized by differential scanning calorimetry (DSC), thermogravimetric (TG) analysis, and powder X-ray diffractometry (PXRD). From the DSC and TG data, it was confirmed that Form 2 is \( {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) methanol solvate, Form 3 is 1 methanol solvate, Form 4 is \( 1{\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) ethanol solvate, and Form 5 is \( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate. The PXRD patterns of five crystal forms were different, respectively. In the dissolution studies in pH 6.8 ± 0.05 buffer at 37 ± 0.5 °C, the solubility of Form 2 was the highest. And the dissolution rate at 5 min in water decreased in rank order: Form 2 > Form 4 > Form 1 > Form 3 > Form 5. After storage of 3 months at 2 °C, 24 % relative humidity, Form 1, Form 2, Form 3, and Form 4 were not transformed, but Form 5 (\( 3{\raise0.5ex\hbox{$\scriptstyle 2$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 3$}} \) hydrate) was transformed to dihydrate.






Similar content being viewed by others
References
Chadha R, Arora P, Garg M, Bhandari S, Jain DS. Thermoanalytical and spectroscopic studies on different crystal forms of nevirapine. J Therm Anal Calorim. 2013;111:2133–42.
Perlovich GL, Blokhina SV, Manin NG, Volkova TV, Tkachev VV. Polymorphism and solvatomorphism of bicalutamide. J Therm Anal Calorim. 2013;111:655–62.
Haleblian J. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J Pharm Sci. 1975;64:1269–88.
Haleblian J, McCrone W. Pharmaceutical applications of polymorphism. J Pharm Sci. 1969;58:911–29.
Shin JY, Sohn YT. Solid state of a new flavonoid derivative DA-6034. J Therm Anal Calorim. 2014;115:2457–61.
Drebushchak VA, Drebushchak TN, Boldyreva EV. New interpretation of heat effects in polymorphic transitions. J Therm Anal Calorim. 2013;113:419–24.
Maria TMR, Castro RAE, Silva MR, Ramos ML, Justino LLG, Burrows HD, Canotilho J, Eusebio MES. Polymorphism and melt crystallisation of racemic betaxolol, a β-adrenergic antagonist drug. J Therm Anal Calorim. 2013;111:2171–8.
Kuhnert-Brandstätter M, Burger A. Untersuchungen zum Aufloesungsverhalten polymorpher, pseudopolymorpher and amorpher Phasen von Arzneimitteln. Pharm Ind. 1972;34:187–90.
Liu W, Dang L, Wei H. Thermal, phase transition, and thermal kinetics studies of carbamazepine. J Therm Anal Calorim. 2013;111:1999–2004.
Szterner P, Legendre B, Sghaier M. Thermodynamic properties of polymorphic forms of theophylline. Part I: DSC, TG, X-ray study. J Therm Anal Calorim. 2010;99:325–35.
FDA. Q6A international conference on harmonization; guidance on Q6A specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances, Federal Register. vol 65. 2000. p. 83041–63.
Seo HO, Sohn YT. Crystal transformation of a flavonoid derivative DA-6034. J Therm Anal Calorim. 2015;120:749–57.
Zhang GGZ, Law D, Scmitt EA, Qiu Y. Phases transformation considerations during process development and manufacture of solid oral dosage forms. Adv Drug Deliv Rev. 2004;56:371–90.
Otsuka M. Effects of environmental temperature and compression energy on polymorphic transformation during tableting. Drug Dev Ind Pharm. 1993;19:2241–69.
Griesser UJ, Weigand D, Rollinger JM, Haddow M, Gstrein E. The crystal polymorphs of metazachlor. J Therm Anal Calorim. 2004;77:511–22.
Dichi E, Legendre B, Sghaier M. Physico-chemical characterization of a new polymorph of caffeine. J Therm Anal Calorim. 2014;115:1551–61.
Petit S, Mallet F, Petit MN, Coquerel G. Role of structural and macrocrystalline factors in the desolvation behavior of cortisone acetate solvates. J Therm Anal Calorim. 2007;90:39–47.
Brittain HG, Grant DJW. Effects of polymorphism and solid-state solvation on solubility and dissolution rate. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 279–330.
de Oliveira GGG, Ferraz HG, Severino P, Souto E. Analysis of phase transition and dehydration process of nevirapine. J Therm Anal Calorim. 2012;108:53–7.
Terada K, Kurobe H, Ito M, Yoshihashi Y, Yonemochi E, Fujii K, Uekusa H. Polymorphic and pseudopolymorphic transformation behavior of acyclovir based on thermodynamics and crystallography. J Therm Anal Calorim. 2013;113:1261–7.
Nicolai B, Espeau P, Ceolin R, Perrin MA, Zaske L, Giovanni J, Leveiller F. Polymorph formation from solvate desolvation. J Therm Anal Calorim. 2007;90:337–9.
Gana I, Ceolin R, Rietveld IB. Bicalutamide polymorphs I and II. J Therm Anal Calorim. 2013;112:223–8.
Huang LF, Tong WQ. Impact of solid state properties on developability assessment of drug candidates. Adv Drug Deliv Rev. 2004;56:321–34.
Lee MJ, Cho KH, Park HM, Sung HJ, Choi SH, Im WB. Pharmacological profile of DA-6886, a novel 5-HT4 receptor agonist to accelerate colonic motor activity in mice. Eur J Pharmacol. 2014;735:115–22.
Rodriguez-Spong B, Price CP, Jayasankar A, Matzger AJ, Rodriguez-Hornedo N. General principles of pharmaceutical solid polymorphism: a supramolecular perspective. Adv Drug Deliv Rev. 2004;56:241–74.
Brittain HG. Methods for the characterization of polymorphs and solvates. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 227–78.
Shefter E, Higuchi T. Dissolution behavior of crystalline solvated and nonsolvated forms of some pharmaceuticals. J Pharm Sci. 1963;52:781–91.
Botha SA, Caira MR, Guillory JK, Lötter AP. Physical characterization of the methanol solvate of urapidil. J Pharm Sci. 1989;78:28–34.
Suleiman MS, Najib NM. Isolation and physicochemical characterization of solid forms of glibenclamide. Int J Pharm. 1989;50:103–9.
Tros de Ilarduya MC, Martin C, Goni MM, Martinez-Oharriz MC. Dissolution rate of polymorphs and two new pseudopolymorphs of sulindac. Drug Dev Ind Pharm. 1997;23:1087–93.
Giron D, Goldbronn Ch, Mutz M, Pfeiffer S, Piechon Ph, Schwab Ph. Solid state characterization of pharmaceutical hydrates. J Therm Anal Calorim. 2002;68:453–65.
Guillory JK. Generation of polymorphs. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker; 1999. p. 183–226.
Byrn S, Pfeiffer R, Ganey M, Hoiberg C, Poochikian G. Pharmaceutical solids: a strategic approach to regulatory considerations. Pharm Res. 1995;12:945–54.
Acknowledgements
This study was supported by the research grant from Duksung Women’s University (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tak, SR., Sohn, YT. Crystal forms of a new 5-HT4 receptor agonist DA-6886. J Therm Anal Calorim 123, 2477–2483 (2016). https://doi.org/10.1007/s10973-015-4897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4897-1