Abstract
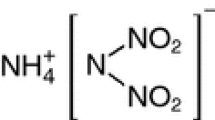
Ammonium dinitramide (ADN) has attracted significant interest as a potential oxidizer for next-generation rocket propellants, since it is a halogen-free alternative to the widely used oxidizer ammonium perchlorate. Because ADN synthesis requires N-nitration generates a very unstable intermediate to form the N–(NO2)2 group, this study used a reaction calorimeter to assess the heat of decomposition during the nitration of potassium sulfamate with various nitration agents (HNO3/H2SO4 and HNO3) by using the HNO3 density between 1.52 and 1.38 gcm−3. The heat of decomposition of potassium sulfamate in HNO3 was found to decrease with decreasing HNO3 density. In contrast, the heat of decomposition did not decrease with density in HNO3/H2SO4, since a significant temperature rise was generated when combining the lower density HNO3 with H2SO4. The heat of decomposition in high-density HNO3 (1.52 gcm−3) was greater than that in the mixed acid, likely because protonation of the potassium sulfate nitrogen by H2SO4 inhibited the subsequent nitration step.








Similar content being viewed by others
References
Bottaro JC. Recent advances in explosives and solid propellants. Chem Ind. 1996;7:249–52.
Jones DEG, Kwok QSM, Vachon M, Badeen C, Ridley W. Characterization of ADN and ADN-based propellants. Propellants Explos Pyrotech. 2005;30:140–7.
Östmark H, Bemm U, Langlet A, Sandén R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energy Mater. 2000;18:123–38.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Schmitt RJ, Bottaro JC, Penwell PE, Bomberger C. Process for forming ammonium dinitramide salt by reaction between ammonia and a nitronium-containing compound. US patent 5,316,749. 1994.
Matsunaga H, Habu H, Miyake A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim. 2014;116:1227–32.
Langlet A, Östmark H, Wingborg N. Method of preparing dinitramidic acid and salts thereof. US patent 5,976,483. 1999.
Hatano H, Onda T, Shiino K, Miyazaki S, Matsuura S. New synthetic method and properties of ammonium dinitramide. Sci Technol Energy Mater. 1996;57:160–5.
Suzuki S, Miyazaki S, Hatano H, Shiino K, Onda T. Synthetic method for forming ammonium dinitramide (ADN). US patent 5,659,080. 1997.
Bottaro JC, Penwell PE, Schmitt RJ. A new synthesis of alkyl-N, N-dinitramines by direct nitration of isocyanates. Synth Commun. 1991;21:945–9.
Yang R, Thakre P, Yang V. Thermal decomposition and combustion of ammonium dinitramide. Combust Explos Shock Waves. 2005;41:657–79.
Löbbecke S, Krause HH, Pfeil A. Thermal analysis of ammonium dinitramide decomposition. Propellants Explos Pyrotech. 1997;22:184–8.
Pavlov AN, Grebennikov VN, Nazina LD, Nazin GM, Manelis GB. Thermal decomposition of ammonium dinitramide and mechanism of anomalous decay of dinitramide salts. Russ Chem Bull. 1999;48:50–4.
Alavia S, Thompson DL. Decomposition pathways of dinitramic acid and the dinitramide ion. J Phys Chem. 2003;119:232–40.
Iwata Y. Evaluation of thermal decomposition hazards by differential adiabatic calorimeter. Sci Technol Energy Mater. 2013;64:160–16574.
Okada K, Funakoshi A, Akiyoshi M, Usuba S, Matsunaga T. Thermal hazard evaluation of ammonium nitrate emulsions by DSC and 1.5 L pressure vessel test. Sci Technol Energy Mater. 2014;75:1–7.
Tanaka K, Kumasaki M, Miyake A. Influence of Mg surface layer for induction period of Grignard reagent formation. J Therm Anal Calorim. 2013;113:1395–401.
Sugie Y, Miyake A. Effects of temperature on nitration of sulfamates. J Therm Anal Calorim. 2014;116:1213–7.
Cupery M. Sulfamic acid: a new industrial chemical. Ind Eng Chem. 1938;30:627–31.
Garret M, Tao T, Jolly WL. The protonation and deprotonation of sulfamide and sulfamate in aqueous solutions. J Phys Chem. 1964;68:824–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugie, Y., Miyake, A. Effect of the density of nitric acid on thermal behavior during sulfamate nitration. J Therm Anal Calorim 121, 275–279 (2015). https://doi.org/10.1007/s10973-015-4564-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4564-6