Abstract
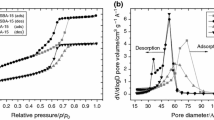
Synthetic silica gels with six different effective diameters of nano-pores (3–30 nm) were loaded with n-hexadecane (cetane) after the elimination of adsorbed water. Kinetics of the solidification and melting of cetane was studied by differential scanning calorimetry (DSC) above the room temperature. Two thermodynamically different states of cetane were found in the samples: the free (bulk)-cetane state and the confined-cetane state. As suspected, the third state of cetane can be amorphous. This has been indicated by the small total transformation heat. The complex crystallization effect of cetane has been found to obey the nucleation-and-growth kinetics and also to depend on the dimensions of confining pores of silica gel. The melting of cetane seems to vary only with the average diameter of silica gel pores, which satisfies the Gibbs–Thompson relation. The presented results validate the applicability of the DSC technique for the porometry. The cetane-medium calibration curve for the silica gel nano-thermoporometry has been determined.









Similar content being viewed by others
References
http://en.wikipedia.org/wiki/Hexadecane; http://en.wikipedia.org/wiki/Silica_gel. 21 Nov 2011.
Landry MR. Thermoporometry by differential scanning calorimetry: experimental considerations and applications. Thermochim Acta. 2005;433:27–50.
Chen LC, Spaepen F. Analysis of calorimetric measurements of grain growth. J Appl Phys. 1991;69:679–88.
Illeková E, Kuhnast FA, Maťko I, Naguet CH. Influence of preannealing on the crystallization of Fe75Si15B10 metallic glass. Mat Sci Eng A. 1996;215:150–6.
Wilde G, Sebright JL, Perepozko JH. Bulk liquid undercooling and nucleation in gold. Acta Mater. 2006;54:4759–69.
Christian JW. The theory of transformations in metals and alloys. New York: Pergamon Press; 1965.
Suñol JJ, Farjas J, Berlanga R, Saurina J. Thermal analysis of a polyethylene glycol (PEG 4000): T-CR-T diagram construction. J Therm Anal Calorim. 2000;61:711–8.
Riikonen J, Salonen J, Lehto VP. Utilising thermoporometry to obtain new insights into nanostructured materials. Review part 1. J Therm Anal Calorim. 2011;105:811–21.
Riikonen J, Salonen J, Lehto VP. Utilising thermoporometry to obtain new insights into nanostructured materials. Review part 2. J Therm Anal Calorim. 2011;105:823–30.
Acknowledgements
Support of the Agency of the Ministry of Education of the Slovak Republic for the Structural Funds of the EU (CEKOMAT II, ITMS 26240120020) is gratefully acknowledged. The research was supported also by the projects VEGA 2/0111/11, 2/0171/09, 2/0099/10 and APVV—0647-10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Illeková, E., Miklošovičová, M., Šauša, O. et al. Solidification and melting of cetane confined in the nanopores of silica gel. J Therm Anal Calorim 108, 497–503 (2012). https://doi.org/10.1007/s10973-011-2113-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2113-5