Abstract
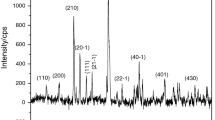
Thermogravimetry combined with evolved gas mass spectrometry has been used to characterise the mineral crandallite CaAl3(PO4)2(OH)5·(H2O) and to ascertain the thermal stability of this ‘cave’ mineral. X-ray diffraction proves the presence of the mineral and identifies the products of the thermal decomposition. The mineral crandallite is formed through the reaction of calcite with bat guano. Thermal analysis shows that the mineral starts to decompose through dehydration at low temperatures at around 139 °C and the dehydroxylation occurs over the temperature range 200–700 °C with loss of the OH units. The critical temperature for OH loss is around 416 °C and above this temperature the mineral structure is altered. Some minor loss of carbonate impurity occurs at 788 °C. This study shows the mineral is unstable above 139 °C. This temperature is well above the temperature in the caves of 15 °C maximum. A chemical reaction for the synthesis of crandallite is offered and the mechanism for the thermal decomposition is given.



Similar content being viewed by others
References
Dumitras D-G, Marincea S, Bilal E, Hatert F. Apatite-(CaOH) in the fossil bat guano deposit from the “dry” Cioclovina Cave, Sureanu Mountains, Romania. Can Miner. 2008;46:431–45.
Hill CA. Cave Minerals. Huntsville: National Speleological Society; 1976.
Moravansky D, Orvosova M. Recent knowledge about the cave minerals of Slovakia. Miner Slov. 2007;39:203–16.
Moravansky D, Zenis P. Guano minerals in some caves of Western and Central Slovakia. Miner Slov. 1997;29:61–72.
Onac BP, Mylroie JE, White WB. Mineralogy of cave deposits on San Salvador Island. Bahamas Carbonates Evaporites. 2001;16:8–16.
White WB. Cave minerals and speleothems. In: Ford DT, Cullingford CHD, editors. The science of speleology. London: Academic Press; 1976. p. 267–327.
Mingaye JCH. Phosphatic deposits in the Jenolan caves. N S W Rep Aust Assoc. 1898;7:111–6.
Mingaye JCH. Phosphatic deposits in the Jenolan caves, New South Wales. Rec Geol Surv N S W. 1899;6:111–6.
Sussmilch CA, Stone WG. Geology of the Jenolan caves district. J Proc R Soc N S W. 1916;49:332–84.
Osborne RAL, Zwingmann H, Pogson RE, Colchester DM. Carboniferous clay deposits from Jenolan caves, New South Wales: implications for timing of speleogenesis and regional geology. Aust J Earth Sci. 2006;53:377–405.
Blanchard FN. Thermal analysis of crandallite. Q J Fla Acad Sci. 1971;34:1–9.
Francisco EaB, Prochnow LI, Motta De Toledo MC, Ferrari VC, Luis De Jesus S. Thermal treatment of aluminous phosphates of the crandallite group and its effect on phosphorus solubility. Sci Agric (Piracicaba, Brazil). 2007;64:269–74.
Guardani R. Thermal transformations and solubility of aluminum phosphates from the states of Para and Maranhao (Brazil). Fertilizantes. 1987;9:6–10.
Ferrari VC, Motta De Toledo MC, Atencio D. Gorceixite from Catalao, Goias, Brazil: rietveld crystal structure refinement, Geologia USP. Ser Cient. 2007;7:25–36.
Guardani R, Valarelli JV, Cekinski E, Pereira SCC. Use of alkaline rocks from Pocos de Caldas (Brazil) and phosphogypsum in the production of potassium fertilizer and sulfur dioxide. Fertilizantes. 1985;7:4–8.
Cejka J, Sejkora J, Bahfenne S, Palmer SJ, Plasil J, Frost RL. Raman spectroscopy of hydrogen-arsenate group (AsO3OH) in solid-state compounds: cobalt mineral phase burgessite Co2(H2O)4[AsO3OH]2·H2O. J Raman Spectrosc. 2011;42:214–8.
Frost RL, Palmer SJ, Kristof J, Horvath E. Dynamic and controlled rate thermal analysis of halotrichite. J Therm Anal Calorim. 2010;99:501–7.
Frost RL, Palmer SJ, Kristof J, Horvath E. Thermoanalytical studies of silver and lead jarosites and their solid solutions. J Therm Anal Calorim. 2010;101:73–9.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing manganese. J Therm Anal Calorim. 2010;100:981–5.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites based upon gallium. J Therm Anal Calorim. 2010;101:195–8.
Kristof J, Frost RL, Palmer SJ, Horvath E, Jakab E. Thermoanalytical studies of natural potassium, sodium and ammonium alunites. J Therm Anal Calorim. 2010;100:961–6.
Palmer SJ, Frost RL. Thermal decomposition of Bayer precipitates formed at varying temperatures. J Therm Anal Calorim. 2010;100:27–32.
Tao Q, He H, Frost RL, Yuan P, Zhu J. Thermal decomposition of silylated layered double hydroxides. J Therm Anal Calorim. 2010;101:153–9.
Frost RL, Hales MC, Martens WN. Thermogravimetric analysis of selected group (II) carbonate minerals, implication for the geosequestration of greenhouse gases. J Therm Anal Calorim. 2009;95:999–1005.
Frost RL, Kristof J, Horvath E. Controlled rate thermal analysis of sepiolite. J Therm Anal Calorim. 2009;98:423–8.
Yang J, Frost RL, Martens WN. Thermogravimetric analysis and hot-stage Raman spectroscopy of cubic indium hydroxide. J Therm Anal Calorim. 2010;100:109–16.
Anthony JW, Bideaux RA, Bladh KW, Nichols MC. Handbook of mineralogy. Tuscon: Mineral Data Publishing; 2000.
Acknowledgements
The financial and infra-structure support of the Queensland University of Technology, Chemistry discipline is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding the instrumentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frost, R.L., Palmer, S.J. & Pogson, R.E. Thermal stability of crandallite CaAl3(PO4)2(OH)5·(H2O) . J Therm Anal Calorim 107, 905–909 (2012). https://doi.org/10.1007/s10973-011-1578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1578-6