Abstract
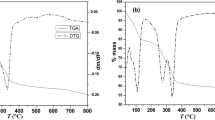
Thermal decomposition kinetics and mechanism of layered double hydroxides (LDHs) intercalating different amounts and types of borate ions were studied using Coats–Redfern integral and Achar differential methods. The results revealed that the Coats–Redfern integral method is more appropriate than the Achar differential method for evaluation of the nonisothermal thermogravimetric decomposition data. The first-order Avrami-Erofe’ev A1 mechanism was found to be the best fitting kinetic model for almost all samples. In addition to the amount and type of the intercalated borate ion, interlayer orientation was also of importance in determining the chemical and thermal stabilities and hence the decomposition kinetics of the studied compounds.


Similar content being viewed by others
References
Vaccari A. Clays and catalysis: a promising future. Appl Clay Sci. 1999;14:161–98.
Lee JD. Concise inorganic chemistry. 4th ed. London: Chapman & Hall; 1991. pp. 359–98.
Coughlin JR. Inorganic borates: chemistry, human exposure, and health and regulatory guidelines. J Trace Elem Exp Med. 1996;9:137–51.
Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002.
Bechara R, D’Huysser A, Fournier M, Forni L, Fornasari G, Trifiro F, Vaccari A. Synthesis and characterization of boron hydrotalcite-like compounds as catalyst for gas-phase transposition of cyclohexanone-oxime. Catal Lett. 2002;82:59–67.
Ay AN. Synthesis and characterization of Mg-Al-layered double hydroxides intercalated by borate anions. PhD Thesis. Ankara; 2007.
Lin JT, Tsai SJ, Cheng S. Beckmann rearrangement of cyclohexanone oxime over borate-pillared LDHs. J Chin Chem Soc-Taip. 1999;46:779–83.
Dahlhoff G, Niederer JPM, Hölderich WF. ε-Caprolactam: new by-product free synthesis routes. Catal Rev. 2001;43:381–441.
Cheng S, Lin JT. Preparation and characterization of borate pillared anionic clays In: Ocelli ML, Robson HE, editors. Expanded clays and microporous solids. New York: van Nostrand Reinhold; 1992. pp. 170–86.
Bhattacharyya A, Hall BD. New triborate-pillared hydrotalcites. Inorg Chem. 1992;31:3869–70.
Li L, Ma S, Liu X, Yue Y, Hui J, Xu R, Bao Y, Rocha J. Synthesis and characterization of tetraborate pillared hydrotalcite. Chem Mater. 1996;8:204–8.
Parker LM, Milestone NB, Newman RH. The use of hydrotalcite as an anion absorbent. Ind Eng Chem Res. 1995;34:1196–202.
Del Arco M, Gutierrez S, Martin C, Rives V, Rocha J. Effect of the Mg:Al ratio on borate (or slicate)/nitrate exchange in hydrotalcite. J Solid State Chem. 2000;151:272–80.
Shi L, Li D, Wang J, Li S, Evans DG, Duan X. Synthesis and smoke-suppressant properties of a borate-intercalated layered double hydroxide. Clays Clay Miner. 2005;53:294–300.
Ay AN, Zümreoglu-Karan B, Mafra L. A simple mechanochemical route to layered double hydroxides: synthesis of hydrotalcite-like Mg-Al-NO3-LDH by manual grinding in a mortar. Z Anorg Allg Chem. 2009;635:1470–5.
Wang L, Lü Z, Li F, Duan X. Study on the mechanism and kinetics of the thermal decomposition of Ni/Al layered double hydroxide nitrate. Ind Eng Chem Res. 2008;47:7211–8.
Ay AN, Zümreoglu-Karan B, Temel A, Mafra L. Layered double hydroxides with interlayer borate anions: a critical evaluation of synthesis methodology and pH-independent orientations in nano-galleries. Appl Clay Sci. Submitted for publication.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Sharp JH, Wentworth SA. Kinetic analysis of thermogravimetric data. Anal Chem. 1969;41:2060–2.
Kolcu O, Zümreoğlu-Karan B. Nonisothermal dehydration kinetics of sodium-light lanthanoid double sulfate monohydrates. Thermochim Acta. 1997;296:135–9.
Moura AO, Prado AGS. Effect of thermal dehydration and rehydration on Na-magadite structure. J Colloid Interface Sci. 2009;330:392–8.
Salentine CG. High-field boron-11 NMR of alkali borates. Aqueous polyborate equilibria. Inorg Chem. 1983;22:3920–4.
Simon JM, Smith RA. Borate raw materials. Glass Technol. 2000;41:169–73.
Weir MR, Kydd RA. Synthesis of metatungstate pillared layered double hydroxides with variable layer composition. Effect of the Mg:Al ratio on the microporous structure. Micropor Mesopor Mat. 1998;20:339–47.
Rives V. Characterization of layered double hydroxides and their decomposition products. Mater Chem Phys. 2002;75:19–25.
Palmer SJ, Spratt HJ, Frost RL. Thermal decomposition of hydrotalcites with variable cationic ratios. J Therm Anal Calorim. 2009;95:123–9.
Wegrzyn A, Rafalska-Lasocha A, Majda D, Dziembaj R, Papp H. The influence of mixed anionic composition of Mg-Al hydrotalcites on the thermal decomposition mechanism based on in situ study. J Therm Anal Calorim. 2010;99:443–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ay, A.N. Importance of the interlayer orientation in borate-intercalating layered double hydroxides. J Therm Anal Calorim 103, 897–902 (2011). https://doi.org/10.1007/s10973-010-1087-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1087-z