Abstract
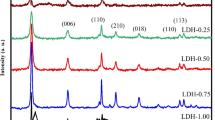
Hydrocalumite (CaAl-Cl-LDH) has the similar structure to layered double hydroxide (LDH). The effects of Na-dodecylsulfate (SDS) on the structure, morphology, and thermal property of CaAl-Cl-LDH have been investigated. Through ion exchange, CaAl-Cl-LDH had been modified with SDS at two concentrations: 0.005 mol L−1 and 0.2 mol L−1. Two different adsorption behaviors were observed through Fourier transform infrared (FTIR) spectra and X-ray diffraction (XRD) patterns. When the SDS concentration was 0.005 mol L−1, surface anion exchange was the major process. When the SDS concentration was 0.2 mol L−1, anion exchange intercalation occurs, with the interlayer distance expanded to 3.25 nm, and the particle morphology from regular hexagons to irregular platelets. The thermal analysis (TG–DTA) showed that dehydration and dehydroxylation occur at a lower temperature when hydrocalumite was intercalated with dodecylsulfate. All these observations revealed that the property of CaAl-Cl-LDH has been changed by SDS modification.




Similar content being viewed by others
References
Stanimirova T, Balek V. Characterization of layered double hydroxide Mg-Al-CO3 prepared by re-hydration of Mg-Al mixed oxide. J Therm Anal Calorim. 2008;94(2):477–81.
Grover K, Komarneni S, Katsuki H. Uptake of arsenite by synthetic layered double hydroxides. Water Res. 2009;43(15):3884–90.
Cavani F, Trifirò F, Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today. 1991;11(2):173–301.
Wei M, Xu XY, He J, et al. Preparation and thermal decomposition study of pyridinedicarboxylate intercalated layered double hydroxides. J Therm Anal Calorim. 2006;85(3):795–800.
Tao Qi, He HP, Frost RL, et al. Thermal decomposition of silylated layered double hydroxides. J Therm Anal Calorim. 2010;101:153–9.
Plank J, et al. Novel organo-mineral phases obtained by intercalation of maleic anhydride-allyl ether copolymers into layered calcium aluminum hydrates. Inorg Chim Acta. 2006;359(15):4901–8.
Hsueh HB, Chen CY. Preparation and properties of LDHs/polyimide nanocomposites. Polymer. 2003;44(4):1151–61.
Xu ZP, Braterman PS. High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J Mater Chem. 2003;13(2):268–73.
Xu ZP, et al. Unusual hydrocarbon chain packing mode and modification of crystallite growth habit in the self-assembled nanocomposites zinc-aluminum-hydroxide oleate and elaidate (cis- and trans-[Zn2Al(OH)6(CH3(CH2)7CHCH(CH2)7COO-)] and magnesium analogues. Chem Mater. 2004;16(14):2750–6.
Xiong Z, Xu Y. Immobilization of palladium phthalocyaninesulfonate onto anionic clay for sorption and oxidation of 2,4,6-trichlorophenol under visible light irradiation. Chem Mater. 2007;19(6):1452–8.
Legrouri A, et al. Removal of the herbicide 2,4-dichlorophenoxyacetate from water to zinc–aluminium–chloride layered double hydroxides. Water Res. 2005;39(15):3441–8.
Zhao H, Nagy K. Dodecyl sulfate-hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. J Colloid Interface Sci. 2004;274(2):613–24.
Baur I, Johnson CA. The solubility of selenate-AFt (3CaO Al2O3 3CaSeO4 37.5H2O) and selenate-AFm (3CaO Al2O3 CaSeO4 xH2O). Cem Concr Res. 2003;33(11):1741–8.
Chrysochoou M, Dermatas D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: literature review and experimental study. J Hazard Mater. 2006;136(1):20–33.
Wu Y, et al. Effective removal of selenate from aqueous solutions by the Friedel phase. J Hazard Mater. 2010;176(1–3):193–8.
You Y, Zhao H, Vance G. Surfactant-enhanced adsorption of organic compounds by layered double hydroxides. Colloids Surf A Physicochem Eng Asp. 2002;205(3):161–72.
Pisson J, et al. Staging of organic and inorganic anions in layered double hydroxides. J Phys Chem B. 2003;107(35):9243–8.
Chao Y, Lee J, Wang S. Preferential adsorption of 2,4-dichlorophenoxyacetate from associated binary-solute aqueous systems by Mg/Al-NO3 layered double hydroxides with different nitrate orientations. J Hazard Mater. 2009;165(1–3):846–52.
Li M, et al. Layered double hydroxides functionalized with anionic surfactant: direct electrochemistry and electrocatalysis of hemoglobin. Electrochim Acta. 2008;53(24):7255–60.
Tao Q, et al. Effect of surfactant concentration on the stacking modes of organo-silylated layered double hydroxides. Appl Clay Sci. 2009;45(4):262–9.
Herrero M, Labajos FM, Rives V. Size control and optimisation of intercalated layered double hydroxides. Appl Clay Sci. 2009;42(3–4):510–8.
Bothe J, Brown P. PhreeqC modeling of Friedel’s salt equilibria at 23°C. Cem Concr Res. 2004;34(6):1057–63.
Zhang H, Wen X, Wang Y. Synthesis and characterization of sulfate and dodecylbenzenesulfonate intercalated zinc-iron layered double hydroxides by one-step coprecipitation route. J Solid State Chem. 2007;180(5):1636–47.
Moyo L, Nhlapo N, Focke W. A critical assessment of the methods for intercalating anionic surfactants in layered double hydroxides. J Mater Sci. 2008;43(18):6144–58.
Acknowledgements
The authors gratefully acknowledge Dr. Xu of the Australian Research Council for the ARC Centre of Excellence for Functional Nanomaterials. This project is financially supported by National Nature Science Foundation of China No. 20907029 and No. 20877053, Shanghai Leading Academic Discipline Project No. S30109.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, P., Shi, H., Xiuxiu, R. et al. Na-dodecylsulfate modification of hydrocalumite and subsequent effect on the structure and thermal decomposition. J Therm Anal Calorim 104, 743–747 (2011). https://doi.org/10.1007/s10973-010-1001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1001-8