Abstract
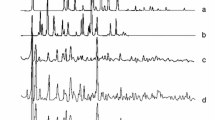
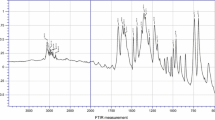
Solid adducts, SbBr3 · L (L = pyridine, 2-, 3- and 4-methylpyridine; abbreviated as Py, 2MPy, 3MPy and 4MPy) were synthesized and characterized by elemental analysis and IR spectroscopy. According to the results coordination of nitrogen of aromatic ring with antimony atom was supposed. Kinetic studies were accomplished by using thermogravimetric data obtained through non-isothermal technique. Determination of activation energy and pre-exponential factor was based on the Coats–Redfern integral Ozawa–Flynn–Wall model-free methods. The kinetics parameters Ea, and log A determined at 5 K min−1 were 150.6 kJ mol−1, 16.0 and 122.0 kJ mol−1, 12.4 for SbBr3 · Py and SbBr3 · 4MPy, respectively.



Similar content being viewed by others
References
Liptay G, Kenessey G, Bihatsi L, Wadsen T, Mink J. Pyridin type complexes of transition-metal-halides. J Therm Anal. 1992;38:899–905.
Liptay G, Kenessey G, Mink J. Pyridine type complexes of transition-metal-halides II: Preparation, characterization and thermal analysis studies of cobalt(II)-bromides and iodides with 2-,3-,4-methylpyridines. Thermochim Acta. 1993;214:71–83.
Mautner FA, Goher MAS. Synthesis, spectroscopic and crystal structure study of di-μ)1,1)-azido-μ(O,O)-nitrato(O-nitrato)tetrakis(3-picoline) aquadicopper(II) and catena-di-μ(1,3)-azido-[di-μ(1,1)-azido-bis(4-picoline)dicopper(II)][Cu2(N3)2(NO3)2(3-picoline)4(H2O)] and Cu(4-picoline)(N3)2. Polyhedron. 1993;12:2823–9.
Sultana N, Tabassum H, Arayne MS. Synthesis and characterization of Cu(I) complexes of triphenylphosphine and 2-methylpyridine. Indian J Chem A. 1994;33:63–4.
Mautner FA, Goher MAS. Spectral and X-ray structure determination of two polymeric complexes of copper(II) azide with 3-picoline and 2-bromopyridine: Cu(3-picoline)2(N3)2 and Cu(2-bromopyridine)(N3)2. Polyhedron. 1992;11:2537–42.
Kiran S, Ravi K, Prem R, Goswami AK. Synthesis and characterization of bis(pentafluorophenyl)antimony(V) cations, [(C6F5)2SbL3]3+. J Fluorine Chem. 2003;122:229–32.
Srinivas N, Kishan MR, Kulkarni SJ, Raghavan KV. Ammoxidation of picolines over modified silicoaluminophosphate molecular sieves. Microporous Mesoporous Mater. 2000;39:125–34.
Das SC. Evaluation of some organic additives for zinc electrowinning from sulphate solutions. Trans Indian Inst Met. 1999;49:781–8.
Das SC, Singh P, Hefter GT. Effects of 2-picoline on zinc electrowinning from acidic sulfate electrolyte. J Appl Electrochem. 1996;26:1245–52.
Dunstan PO, Airoldi C. Adducts of arsenic trihalides with heterocyclic amines. J Chem Eng Data. 1988;33:93–8.
Ptaszynski B. The thermal-decomposition of complex salts of bismuth(III) bromide with hydrobromides of aromatic-amines. Thermochim Acta. 1994;232:137–44.
Pontes FML, Oliveira SF, Espínola JGP, Fonseca MG, Arakaki LNH. Picoline as ligand with antimony trichloride and triiodide adducts. J Therm Anal Calorim. 2004;75:975–88.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881.
Flynn J, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:232–7.
Brown ME, Dollimore D, Galwey AK. Comprehensive chemical kinetics, vol. 22. Amsterdam: Elsevier; 1980.
Ribeiro CA, de Souza WR, Crespi MS, Gomes Neto JA. Non-isothermal kinetic of oxidation of tungsten carbide. J Therm Anal Calorim. 2007;90:801–5.
Logvinenko V. Model-free approach in the study of decomposition kinetics for cluster compounds and coordination compounds. J Therm Anal Calorim. 2008;93:805–9.
Vadim M, Serge B. Modulated thermogravimetry in analysis of decomposition kinetics. Chem Eng Sci. 2005;60:747–66.
Cotas AW, Redfern JP. Kinetics parameters from thermogravimetric data. Nature. 1964;201:68–9.
Green JHS, Kynaston W, Paisley HM. Vibrational spectra of monosubstituted pyridines. Spectrochim Acta. 1963;19:549–64.
Lamba OP, Parihar JS, Jaint YS. Laser-excited Raman and infrared-spectra of alpha-picolines, beta-picolines and gamma-picolines. Indian J Pure Appl Phys. 1983;21:236–42.
Acknowledgments
The authors thank to CAPES and CNPq for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, E.P.S., Botelho, J.R., Oliveira, S.F. et al. Thermal decomposition study of antimony (III) tribromide and aromatic amine adducts. J Therm Anal Calorim 97, 427 (2009). https://doi.org/10.1007/s10973-009-0087-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-009-0087-3