Abstract
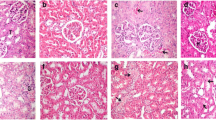
We designed this study to determine possible protective roles of melatonin and carnosine on radiation. Wistar albino rats were divided to five groups. Melatonin, carnosine and combinated version were injected to Group III, IV and V every 48 h in a week. Group II, III, IV and V were exposed to 8 Gy gamma radiation dose arranged for whole-body one hour later second injection. Degenerative and biochemical changes were determined in radiation group as compared to control group. Administration of melatonin, carnosine and their combination ameliorated these parameters. We can conclude melatonin, carnosine and their combination protect kidney against radiation.







Similar content being viewed by others
Data availability
The authors declare that [the/all other] data supporting the findings of this study are available within the article.
References
Shao L, Yang W, Xu R, Zhu S, Huang Y, Li H, Wu X, Yue M, Xiong X, Chen X, Kuang B, Fan G, Zhu Q, Zeng H (2018) Inhibition of mTORC1 signaling protects kidney from irradiation-induced toxicity via accelerating recovery of renal stem-like cells. Stem Cell Res Ther 9:219
Karabulut-Bulan O, Us H, Bayrak BB, Sezen-Us A, Yanardag R (2017) The role of melatonin and carnosine in prevention of oxidative intestinal injury induced by gamma irradiation in rats. Biologia 72:935–945
Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, Pan C, Ten Haken RK, Schultheiss TE (2010) Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 76:S108–S115
Seidensticker M, Burak M, Kalinski T, Garlipp B, Koelble K, Wust P, Antweiler K, Seidensticker R, Mohnike K, Pech M, Ricke J (2015) Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol 38:213–221
Bouillet T, Ali AM, Thariat J (2012) Radiation-induced nephropathy. Bull Cancer 99:389–396
Claustrat B, Leston J (2015) Melatonin: physiological effects in humans. Neurochirurgie 61:77–84
Boldyrev A, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93:1803–1845
Rahman A, Hasan AU, Kobori H (2019) Melatonin in chronic kidney disease: a promising chronotherapy targeting the intrarenal renin-angiotensin system. Hypertens Res 42:920–923
Chen L, Han Z, Shi Z, Liu C, Lu Q (2021) Melatonin alleviates renal injury in mouse model of sepsis. Front Pharmacol 12:697643
Dun RL, Lan TY, Tsai J, Mao JM, Shao YQ, Hu XH, Zhu WJ, Qi GC, Peng Y (2022) Protective effect of melatonin for renal ischemia-reperfusion injury: a systematic review and meta-analysis. Front Physiol 12:791036
Zhang C, Suo M, Liu L, Qi Y, Zhang C, Xie L, Zheng X, Ma C, Li J, Yang J, Bu P (2021) Melatonin alleviates contrast-induced acute kidney injury by activation of Sirt3. Oxid Med Cell Longev. https://doi.org/10.1155/2021/6668887
Raza Z, Naureen Z (2020) Melatonin ameliorates the drug induced nephrotoxicity: molecular insights. Nefrologia (Engl Ed) 40:12–25
Hong TS, Briscese K, Yuan M, Deshpande K, Aleksunes LM, Brunetti L (2021) Renoprotective effects of melatonin against vancomycin-related acute kidney injury in hospitalized patients: a retrospective cohort study. Antimicrob Agents Chemother 65:e0046221
Ozdemir O, Okumus NO, Gursel B, Meydan AD, Meydan BC, Yapici O, Yilmaz Rakici S (2022) The effect of melatonin and carnitine on radiation nephropathy.J Radiat Cancer Res. https://www.journalrcr.org/temp/JRadiatCancerRes000-4490483_122824.pdf
Kilis-Pstrusinska K (2020) Carnosine and kidney diseases: what we currently know? Curr Med Chem 27:1764–1781
Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Köppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327
Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, Matsumura Y (2006) Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther 319:640–647
Fernández M, Medina A, Santos F, Carbajo E, Rodríguez J, Álvarez J, Cobo A (2001) Exacerbated inflammatory response induced by insulin-like growth factor-I treatment in rats with ischemic acute renal failure. J Am Soc Nephrol 12:1900–1907
Barker SB (1944) The direct colorimetric determination of urea in blood and urine. J Biol Chem 152:453–463
Bonsnes RW, Taussky HH (1945) On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581–591
Beutler E (1975) Glutathione in red cell metabolism, a manual of biochemical methods. Grune and Stratton, New York
Ledwozyw A, Michalak J, Stepień A, Kadziołka A (1986) The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clin Chim Acta 155:275–283
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Method Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Mylroie AA, Collins H, Umbles C, Kyle J (1986) Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 82:512–520
Wendel A (1981) Glutathione peroxidase. Method Enzymol 77:325–333
Habig WH, Jakoby WB (1981) Assays of differentiation of glutathione S-transferases. Method Enzymol 77:398–405
Furlong CE, Richter RJ, Seidel SL, Motulsky AG (1988) Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am J Hum Genet 43:230–238
Geyer JW, Dabich D (1971) Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem 39:412–417
Karker H (1964) Method for estimation of serum adenosine deaminase. Scand J Clin Lab Invest 16:570–574
Ridderstap AS, Bonting SL (1969) Na+-K+-activated ATPase and exocrine pancreatic secretion in vitro. Am J Physiol 217:1721–1727
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Zhang Y, Chen J, Ji H, Xiao ZG, Shen P, Xu LH (2018) Protective effects of Danshen injection against erectile dysfunction via suppression of endoplasmic reticulum stress activation in a streptozotocin-induced diabetic rat model. BMC Complement Altern Med 18:343
Wei H, Frenkel K (1991) In vivo formation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Res 51:4443–4449
Lowry OH, Rosebrough NJ, Farr AL, Randall J (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Kucuktulu E (2012) Protective effect of melatonin against radiation induced nephrotoxicity in rats. Asian Pac J Cancer Prev 13:4101–4105
Canyilmaz E, Uslu GH, Bahat Z, Kandaz M, Mungan S, Haciislamoglu E, Mentese A, Yoney A (2016) Comparison of the effects of melatonin and genistein on radiation-induced nephrotoxicity: Results of an experimental study. Biomed Rep 4:45–50
Mehrvar S, la Cour MF, Medhora M, Camara AKS, Ranji M (2019) Optical metabolic imaging for assessment of radiation-induced injury to rat kidney and mitigation by lisinopril. Ann Biomed Eng 47:1564–1574
Aratani S, Tagawa M, Nagasaka S, Sakai Y, Shimizu A, Tsuruoka S (2018) Radiation-induced premature cellular senescence involved in glomerular diseases in rats. Sci Rep 8:16812
Abdel-Magied N, Elkady AA (2019) Possible curative role of curcumin and silymarin against nephrotoxicity induced by gamma-rays in rats. Exp Mol Pathol 111:104299
Haeri SA, Rajabi H, Fazelipour S, Hosseinimehr SJ (2014) Carnosine mitigates apoptosis and protects testicular seminiferous tubules from gamma-radiation-induced injury in mice. Andrologia 46:1041–1046
Kunwar A, Bag PP, Chattopadhyay S, Jain VK, Priyadarsini KI (2011) Anti-apoptotic, anti-inflammatory, and immunomodulatory activities of 3,3’-diselenodipropionic acid in mice exposed to whole body γ-radiation. Arch Toxicol 85:1395–1405
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 31:332–336
Sert C, Çelik MS (1996) Radyasyondan koruyucu ajanlar. Turkiye Klinikleri J Med Sci 16:292–298
Somosy Z, Horváth G, Telbisz Á, Réz G, Pálfia Z (2002) Morphological aspects of ionizing radiation response of small intestine. Micron 33:167–178
Baykara B, Tekmen I, Pekcetin C, Ulukus C, Tuncel P, Sagol O, Ormen M, Ozogul C (2009) The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochem 111:42–51
Marzook EA, Marzook FA, Abd El Moneim AE (2020) Radioprotective and anti-diabetic effects of Costus speciosus and carnosine. Trop J Pharm Res 19:121–127
Aziz MM, Eid NI, Nada AS, Amin NE, Ain-Shoka AA (2018) Possible protective effect of the algae spirulina against nephrotoxicity induced by cyclosporine A and/or gamma radiation in rats. Environ Sci Pollut Res Int 25:9060–9070
Fouad D, Alhatem H, Abdel-Gaber R, Ataya F (2019) Hepatotoxicity and renal toxicity induced by gamma-radiation and the modulatory protective effect of Ficus carica in male albino rats. Res Vet Sci 125:24–35
Altintas R, Polat A, Parlakpinar H, Vardi N, Beytur A, Oguz F, Sagir M, Yildiz A, Duran ZR (2014) The effect of melatonin on acetylsalicylic acid-induced kidney and testis damage. Hum Exp Toxicol 33:383–395
Hipkiss AR, Brownson C, Bertani MF, Ruiz E, Ferro A (2002) Reaction of carnosine with aged proteins: another protective process? Ann N Y Acad Sci 959:285–294
Ezz MK, Ibrahim NK, Said MM, Farrag MA (2018) The beneficial radioprotective effect of tomato seed oil against gamma radiation-induced damage in male rats. J Diet Suppl 15:923–938
Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci 74:3863–3881
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B (2018) Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions.Molecules 23:509
Hoffman JR, Varanoske A, Stout JR (2018) Effects of β-alanine supplementation on carnosine elevation and physiological performance. Adv Food Nutr Res 84:183–206
Zhao W, Robbins ME (2009) Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem 16:130–143
Sauerhöfer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N, Floege J, Kriz W, van der Woude F, Moeller MJ (2007) L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432
Scheuer C, Pommergaard HC, Rosenberg J, Gögenur I (2014) Melatonin’s protective effect against UV radiation: a systematic review of clinical and experimental studies. Photodermatol Photoimmunol Photomed 30:180–188
Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ (2001) Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res 30:65–74
Antolín I, Rodríguez C, Saínz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A (1996) Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J 10:882–890
Serhatlioglu S, Gursu MF, Gulcu F, Canatan H, Godekmerdan A (2003) Levels of paraoxonase and arylesterase activities and malondialdehyde in workers exposed to ionizing radiation. Cell Biochem Funct 21:371–375
Efe TH, Ertem AG, Altunoglu A, Koseoglu C, Erayman A, Bilgin M, Kurmuş Ö, Aslan T, Bilge M (2016) Serum paraoxonase levels are correlated with impaired aortic functions in patients with chronic kidney disease. Acta Cardiol Sin 32:75–80
Cruz FF, Pereira TCB, Altenhofen S, da Costa KM, Bogo MR, Bonan CD, Morrone FB (2019) Characterization of the adenosinergic system in a zebrafish embryo radiotherapy model. Comp Biochem Physiol C Toxicol Pharmacol 224:108572
Alkis H, Demir E, Taysi MR, Sagir S, Taysi S (2021) Effects of Nigella sativa oil and thymoquinone on radiation-induced oxidative stress in kidney tissue of rats. Biomed Pharmacother 139:111540
Shadyro OI, Yurkova IL, Kisel MA (2002) Radiation-induced peroxidation and fragmentation of lipids in a model membrane. Int J Radiat Biol 78:211–217
Tain YL, Chen CC, Lee CT, Kao YH, Sheen JM, Yu HR, Huang LT (2013) Melatonin regulates L-arginine transport and NADPH oxidase in young rats with bile duct ligation: role of protein kinase C. Pediatr Res 73:395–401
Kaločayová B, Kovačičová I, Radošinská J, Tóthová Ľ, Jagmaševič-Mézešová L, Fülöp M, Slezák J, Babál P, Janega P, Vrbjar N (2019) Alteration of renal Na,K-ATPase in rats following the mediastinal γ-irradiation. Physiol Rep 7:e13969
Gariballa SE, Sinclair AJ (2000) Carnosine: physiological properties and therapeutic potential. Age Ageing 29:207–210
García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res 56:225–237
Yay A, Akkuş D, Yapıslar H, Balcıoglu E, Sonmez MF, Ozdamar S (2014) Antioxidant effect of carnosine treatment on renal oxidative stress in streptozotocin-induced diabetic rats. Biotech Histochem 89:552–557
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61:253–278
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51:1–16
Sener G, Kabasakal L, Atasoy BM, Erzik C, Velioğlu-Oğünç A, Cetinel S, Gedik N, Yeğen BC (2006) Ginkgo biloba extract protects against ionizing radiation-induced oxidative organ damage in rats. Pharmacol Res 53:241–252
Galijasevic S, Abdulhamid I, Abu-Soud HM (2008) Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry 47:2668–2677
Carroll L, Karton A, Radom L, Davies MJ, Pattison DI (2019) Carnosine and carcinine derivatives rapidly react with hypochlorous acid to form chloramines and dichloramines. Chem Res Toxicol 32:513–525
Acknowledgements
This study was supported by the Scientific Research Projects Coordination Unit of Istanbul University. Project Number: 57797.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to this research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turkyilmaz, I.B., Us, H., Sezen Us, A. et al. Protective effect of melatonin and carnosine against radiation induced kidney injury. J Radioanal Nucl Chem 331, 3551–3561 (2022). https://doi.org/10.1007/s10967-022-08419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08419-6