Abstract
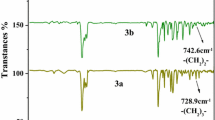
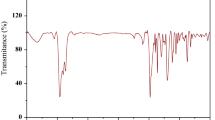
P-carboxyphenyl azo calix[4]arene phosphate derivative (P-CACPD) was successfully synthesized using calix[4]arene as raw material. The P-CACPD was characterized by FT-IR and 1H-NMR. The effects of pH, extraction time and temperature on the extraction of U(VI) by P-CACPD were investigated. The results show that pH value, extraction time and temperature have an effect on the extraction of U(VI) by P-CACPD. When the initial pH is 5, temperature is 25 °C, extraction time is 2 h, the extraction rate of U(VI) by P-CACPD reaches the maximum. Both kinetics parameters and thermodynamic parameters of the extraction process were calculated. The calculated data indicate that the extraction process of uranium by P-CACPD is an exothermic spontaneous process, which well fits with the pseudo-second-order kinetic model.








Similar content being viewed by others
References
Peng GW, Ding DX, Xiao FZ, Wang XL, Hun N, Wang YD, Dai YM, Cao Z (2014) Adsorption of uranium ions from aqueous solution by amine-group functionalized magnetic Fe3O4 nanoparticle. J Radioanal Nucl Chem 301(3):781–788
Fangzhu X, Guowen P, Dexin D, Yimin D (2015) Preparation of a novel biosorbent ISCB and its adsorption and desorption properties of uranium ions in aqueous solution. J Radioanal Nucl Chem 306(2):349–356
Xie S, Yang J, Chen C, Zhang X, Wang Q, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99(1):126–133
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366(1):165–172
Mellah A, Chegrouche S, Barkat M (2007) The precipitation of ammonium uranyl carbonate (AUC): thermodynamic and kinetic investigations. Hydrometallurgy 85(2–4):163–171
Armagan AF, Mustafa S (2007) Solid phase extraction and preconcentration of uranium(VI) and thorium(IV) on Duolite XAD761 prior to their inductively coupled plasma mass spectrometric determination. Talanta 72(1):187–192
Yaftian MR, Taheri R, Zamani AA, Matt D (2004) Thermodynamics of the solvent extraction of thorium and europium nitrates by neutral phosphorylated ligands. J Radioanal Nucl Chem 262(2):455–459
Luke C, Ewa C, Lorraine S, Koovila N (2003) Supported-liquid membrane extraction as a selective sample preparation technique for monitoring uranium in complex matrix samples. J Sep Sci 26(6–7):601–608
Dietz ML, Horwitz EP, Sajdak LR, Chiarizia R (2001) An improved extraction chromatographic resin for the separation of uranium from acidic nitrate media. Talanta 54(6):1173–1184
Ladeira ACQ, Morais CA (2005) Uranium recovery from industrial effluent by ion exchange—column experiments. Miner Eng 18(13):1337–1340
Saeid HM, Heidar R, Reza YH (2006) Synergistic flotation of U(VI)–alizarin complex with some diamines followed by spectrophotometric determination of U(VI) using 4,4′-diaminophenylmethane. Anal Chim Acta 559(2):181–185
Oshita K, Sabarudin A, Takayanagi T, Oshima M, Motomizu S (2009) Adsorption behavior of uranium(VI) and other ionic species on cross-linked chitosan resins modified with chelating moieties. Talanta 79(4):1031–1035
Qiu PY, Fangzhu X, Suya H, Cheng W, Wen PG, Yong L (2017) Synthesis of the p-tert-butyl calix[4] arene symmetrical sulfide derivatives and its extraction properties towards U(VI) from aqueous solution. J Radioanal Nucl Chem 314(3):2137–2143
Beer PD, Drew Michael G B, Kan M, Leeson PB, Ogden MI, Gareth W (1996) Lanthanide structures, coordination, and extraction investigations of a 1,3-bis(diethyl amide)-substituted calix[4]arene ligand. Inorg Chem 35(8):2202–2211
Sessler JL, Melfi PJ, Dan PG (2006) Uranium complexes of multidentate N-donor ligands. Coord Chem Rev 250(7–8):816–843
Maria K, Françoise A-N, Véronique H-B, Igor S, Vitaly K (2010) Novel phosphorylated calixarenes for the recognition of f-elements. J Incl Phenom Macrocycl Chem 66(1–2):113–123
Keisuke O, Masayuki Y, Katsutoshi I, Takehisa Y, Masahiro G, Fumiyuki N, Seiji S, Takeshi N (1995) Solvent extraction of trivalent rare earth metal ions with carboxylate derivatives of calixarenes. Anal Sci 11(6):893–902
Cedricb D, Dominique M, Anthony H (2000) Coordination chemistry of calix-phosphanes: cooperativity in the assembly of a tetragold calixarene complex. Eur J Inorg Chem 2000(5):831–834
Gutsche CD, Iqbal M, Nam KS, See K, Alam I (1988) Conformational and complexational characteristics of calixarenes. Pure Appl Chem 60(4):483–488
David GC, Muzaffer I, Donald S (1986) Calixarenes 19: syntheses procedures for p-tert-butylcalix[4]arene. J Org Chem 51(5):742–745
David GC, Gin LL (1986) Calixarenes 12: the synthesis of functionalized calixarenes. Tetrahedron 42(6):1633–1640
Chonggang FU, Ailin LIU, Liyun ZHANG, Yanfang ZHAO (2003) Synthesis and spectroscopic proper ties of carboxy lazo calix[4]arene derivatives. Chem World 44(3):151–154
Tang J, Sun Y, Li L, Xu Z, Lv Z (2015) Research on synthesis of new azo calix[4]arene and its dyeing properties, p 02016
Grynszpan F, Bialio SE (1992) Source of the intraannular hydrogens in the dehydroxylation of calix [4] arene diethyl phosphate ester derivatives. J Phys Org Chem 5(3):155–159
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63(7):1165–1169
Savvin SB (1961) Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta 8(9):673–685
Anirudhan TS, Divya L, Suchithra PS (2009) Kinetic and equilibrium characterization of uranium(VI) adsorption onto carboxylate-functionalized poly(hydroxyethylmethacrylate)-grafted lignocellulosics. J Environ Manage 90(1):549–560
Günther M (1998) Aquatic chemistry of uranium. FOG: Freiberg Online Geoscience 1
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51574152, 11705085), the China Postdoctoral Science Foundation (No. 2016M602418), the Natural Science Foundation of Hunan Province (Nos. 2017JJ2232, 2017JJ3262, 2017JJ4009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Xiao, Fz., Pu, Yq. et al. Preparation of p-carboxyphenyl azo calix[4]arene phosphate derivative and its extraction properties toward uranium(VI). J Radioanal Nucl Chem 317, 1235–1241 (2018). https://doi.org/10.1007/s10967-018-6000-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6000-4