Abstract
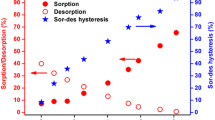
The sorption and desorption of uranium(VI) on GMZ bentonite was investigated as functions of contact time, pH, ionic strength, foreign ions, HSs and initial U(VI) using batch experiments. It is revealed that the sorption strongly depended on pH, cations (Li+, Na+ and K+) and anions (CO3 2−, SO4 2− and PO4 3−). HA benefits the sorption at pH <7.0, while inhibited the sorption at higher pH. Moreover, the hysteresis occurred in the sorption–desorption process in the presence/absence of humic substances. The results could provide data base for the safety assessment of the deep geological disposal repository of high radioactive waste.













Similar content being viewed by others
References
Lu S, Xu H, Wang M, Song X, Liu Q (2012) Sorption of Eu(III) onto Gaomiaozi bentonite by batch technique as a function of pH, ionic strength, and humic acid. J Radoanal Nucl Chem 292:889–895
Jia W, Lu S (2014) Effect of pH, foreign ions and temperature on radionickel sorption onto bentonite from Inner Mongolia, China. J Radioanal Nucl Chem 299:1417–1426
Wang XK, Chen CL, Zhou X, Tan XL, Hu WP (2005) Diffusion and sorption of U(VI) in compacted bentonite studied by a capillary method. Radiochim Acta 93:273–278
Sun Y, Li J, Wang X (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Dong Y, Liu Z, Li Y (2011) Effect of pH, ionic strength, foreign ions and humic substances on Th(IV) sorption to GMZ bentonite studied by batch experiments. J Radioanal Nucl Chem 289:257–265
Yang S, Li J, Lu Y, Chen Y, Wang X (2009) Sorption of Ni(II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl Radiat Isot 67:1600–1608
Wang S, Hu J, Li J, Dong Y (2009) Influence of pH, soil humic/fulVIc acid, ionic strength, foreign ions and addition sequences on adsorption of Pb(II) onto GMZ bentonite. J Hazard Mater 167:44–51
Li J, Hu J, Sheng G, Zhao G, Huang Q (2009) Effect of pH, ionic strength, foreign ions and temperature on the adsorption of Cu(II) from aqueous solution to GMZ bentonite. Colloids Surf A 349:195–201
Smith GM, Smith KL, Kowe R, Perez-Sanchez D, Thorne M, Thiry Y, Read D, Molinero J (2014) Recent developments in assessment of long-term radionuclide behaVIor in the geosphere-biosphere subsystem. J Environ Radioact 131:89–109
Müller K, Foerstendorf H, Brendler V, Rossberg A, Stolze K, Gröschel A (2013) The surface reactions of U(VI) on γ-Al2O3—in situ spectroscopic evaluation of the transition from sorption complexation to surface precipitation. Chem Gelo 357:75–84
Mei H, Tan X, Yu S, Ren X, Chen C, Wang X (2015) Effect of silicate on U(VI) sorption to γ-Al2O3: batch and EXAFS studies. Chem Eng J 269:371–378
Gao Y, Shao Z, Xiao Z (2014) U(VI) sorption on illite: effect of pH, ionic strength, humic acid and temperature. J Radioanal Nucl Chem 303:867–876
Marques FM, Baeyens B, Dähn R, Scheinost AC, Bradbury MH (2012) U(VI) sorption on montmorillonite in the absence and presence of carbonate: a macroscopic and microscopic study. Geochim Cosmochim Acta 93:262–277
Cheng W, Ding C, Sun Y, Wang X (2015) Fabrication of fungus/attapulgite composites and their removal of U(VI) from aqueous solution. Chem Eng J 269:1–8
Xiao J, Chen Y, Zhao W, Xu J (2013) Sorption behaVIor of U(VI) onto Chinese bentonite: effect of pH, ionic strength, temperature and humic acid. J Mol Liq 188:178–185
Zhu W, Liu Z, Chen L, Dong Y (2011) Sorption of uranium(VI) on Na-attapulgite as a function of contact time, solid content, pH, ionic strength, temperature and humic acid. J Radioanal Nucl Chem 289:781–788
Mishra S, Maity S, Bhalke S, Pandit GG, Puranik VD, Kushwaha HS (2012) Thermodynamic and kinetic investigations of uranium adsorption on soil. J Radioanal Nucl Chem 294:97–102
Dong W, Tokunaga TK, Davis JA, Wan J (2012) Uranium(VI) adsorption and surface complexation modeling onto background sediments from the F-Area Savannah River Site. Environ Sci Technol 46:1565–1571
Dong W, Wan J (2014) Additive surface complexation modeling of uranium(VI) adsorption onto quartz-sand dominated sediments. Environ Sci Technol 48:6569–6577
Maher K, Bargar JR, Brown GE Jr (2013) Environmental speciation of actinides. Inorg Chem 52:3510–3532
Ren X, Yang S, Hu F, He B, Xu J, Tan X, Wang X (2013) Microscopic level investigation of Ni(II) sorption on Na-rectorite by EXAFS technique combined with statistical F-tests. J Hazard Mater 252–253:2–10
Tan X, Fan Q, Wang X, Grambow B (2009) Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS studies. Environ Sci Technol 43:3115–3121
Tan X, Fang M, Li J, Lu Y, Wang X (2009) Adsorption of Eu(III) onto TiO2: effect of pH, concentration, ionic strength and soil fulvic acid. J Hazard Mater 168:458–465
Niu Z, Fan Q, Wang W, Xu J, Chen L, Wu W (2009) Effect of pH, ionic strength and humic acid on the sorption of uranium(VI) to attapulgite. Appl Radiat Isot 67:1582–1590
Dong C, Chen W, Liu C, Liu Y, Liu H (2014) Synthesis of magnetic chitosan nanoparticle and its adsorption property for humic acid from aqueous solution. Colloids Surf A 446:179–189
Xu D, Tan XL, Chen CL, Wang XK (2008) Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: effect of pH, ionic strength, foreign ions and temperature. Appl Clay Sci 41:37–46
Zaitan H, Bianchi D, Achak O, Chafik T (2008) A comparative study of the adsorption and desorption of ο-xylene onto bentonite clay and alumina. J Hazard Mater 153:852–859
Shan C, Ma Z, Tong M (2014) Efficient removal of trace antimony(III) through adsorption by hematite modified magnetic nanoparticles. J Hazard Mater 268:229–236
Zhou L, Liu Z, Jia Y, Peng J, Al-Zaini E (2014) Competitive adsorption of uranium(VI) and thorium(IV) ions from aqueous solution using triphosphate-crosslinked magnetic chitosan resins. J Radioanal Nucl Chem 302:331–340
Malamis S, Katsou E (2013) A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms. J Hazard Mater 252–253:428–461
Sheng G, Hu J, Jin H, Yang S, Ren X, Li J, Chen Y, Wang X (2010) Effect of humic acid, fulvic acid, pH, ionic strength and temperature on 63Ni(II) sorption to MnO2. Radiochim Acta 98:1–9
Sun Y, Qi W, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027
Du Y, Yin Z, Wu H, Li P, Qi W, Wu W (2015) Sorption of U(VI) on magnetic illite: effects of pH, ions, humic substances and temperature. J Radioanal Nucl Chem 304:793–804
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Payne TE, Davis JA, Lumpkin GR, Chisari R, Waite TD (2004) Surface complexation model of uranyl sorption on Georgia kaolinite. Appl Clay Sci 26:151–162
Fan QH, Hao LM, Wang CL, Zheng Z, Liu CL, Wu WS (2014) The adsorption behavior of U(VI) on granite. Environ Sci Process Impacts 16:534–541
Papelis C, Hayes KF (1996) Distinguishing between interlayer and external sorption sites of clay minerals using X-ray absorption spectroscopy. Colloid Surf A 107:89–96
Hayes KF, Papelis C, Leckie JO (1988) Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interfaces. J Colloid Interf Sci 125:717–726
Guo Z, Yan C, Xu J, Wu W (2009) Sorption of U(VI) and phosphate on γ-alumina: Binary and ternarysorption systems. Colloids Surf A 336:123–129
Grabias E, Gładysz-Płaska A, Książek A, Majdan M (2014) Efficient uranium immobilization on red clay with phosphates. Environ Chem Lett 12:297–301
Del Nero M, Galindo C, Barillon R, Made B (2011) TRLFS evidence for precipitation of uranyl phosphate on the surface of alumina: environmental implications. Environ Sci Technol 45:3982–3988
Singh A, Catalano JG, Ulrich KU, Giammar DE (2012) Molecular-scale structure of uranium(VI) immobilized with goethite and phosphate. Environ Sci Technol 46:6594–6603
Gückel K, Rossberg A, Brendler V, Foerstendorf H (2012) Binary and ternary surface complexes of U(VI) on the gibbsite/water interface studied by vibrational and EXAFS spectroscopy. Chem Gelo 326–327:27–35
Wu W, Fan Q, Xu J, Niu Z, Lu S (2007) Sorption-desorption of Th(IV) on attapulgite: effects of pH, ionic strength and temperature. Appl Clay Sci 65:1108–1114
Tao ZY, Zhang J, Zhai JJ (1999) Characterization and differentiation of humic acids and fulvic acids in soils from various regions of China by nuclear magnetic resonance spectroscopy. Anal Chim Acta 395:199
Hu J, Shao DD, Chen CL, Sheng GD, Li J, Wang XK, Nagatsu M (2010) Plasma-Induced grafting of cyclodextrin onto multiwall carbon nanotube/iron oxides for adsorbent application. J Phys Chem B 114:6779
Pan DQ, Fan QH, Ding KF, Ping L, Yan L, Tao Y, Jiang X, Wu WS (2011) The sorption mechanisms of Th(IV) on attapulgite. Sci China Chem 54:1138–1147
Wang X, Xu D, Chen L, Tan X, Zhou X, Ren A, Chen C (2006) Sorption and complexation of Eu(III) on alumina: effects of pH, ionic strength, humic acid and chelating resin on kinetic dissociation study. Appl Clay Sci 64:414–421
Acknowledgments
We would gratefully thank Pro. Chunli Liu from Peking University for providing the GMZ bentonite sample. Financial support from the Radiochemistry 909 profect in the China Academy of Engineering Physics is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Wang, X., Huang, Z. et al. Sorption and desorption of uranium(VI) on GMZ bentonite: effect of pH, ionic strength, foreign ions and humic substances. J Radioanal Nucl Chem 308, 877–886 (2016). https://doi.org/10.1007/s10967-015-4513-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4513-7