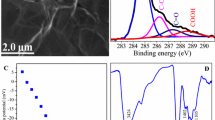
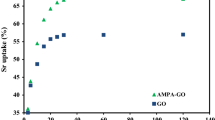
Abstract
The graphene oxides were applied as adsorbents to remove 90Sr(II) ions from aqueous solutions under different experimental conditions. The results showed that 90Sr(II) sorption was mainly dominated by ion exchange and outer-sphere surface complexation. The maximum sorption capacity (C smas) was calculated to be 3.84 × 10−4 mol/g at pH 5.8 and 20 °C, which was much higher than that of 90Sr(II) on other today’s materials. The thermodynamic parameters suggested that the sorption was an endothermic and spontaneous process. The theoretical calculation results indicated that COH and COC groups contributed to the coordination of Sr(II) ions.







Similar content being viewed by others
References
Tan XL, Fan QH, Wang XK, Grambow B (2009) Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS study. Environ Sci Technol 43:3115–3121
Yu SJ, Wang XX, Tan XL, Wang XK (2015) Sorption of radionuclides from aqueous systems onto graphene oxide-based materials: a review. Inorg Chem Front 2:593–612
Fan QH, Tan XL, Li JX, Wang XK, Wu WS, Montavon G (2009) Sorption of Eu(III) on attapulgite studied by batch, XPS and EXAFS techniques. Environ Sci Technol 43:5776–5782
Han RP, Zhang JH, Zou WH, Xiao HJ, Shi J, Liu HM (2006) Biosorption of copper(II) and lead(II) from aqueous solution by chaff in a fixed-bed column. J Hazard Mater 133:262–268
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283
Tan X, Ren X, Chen C, Wang X (2014) Analytical approaches to the speciation of lanthanides on solid-water interfaces. TrAC-Trends Anal Chem 61:107–132
Zhang ZL, Li L (2015) synthesis and characterization of whisker surface imprinted polymer and selective solid-phase extraction of trace Sr(II) from environment aqueous solution. Desalin Water Treat 54:2441–2451
Wang TH, Payne TE, Harrison JJ, Teng SP (2015) Interactions involving strontium and various organic acids on the surface of bentonite (MX-80). J Radioanal Nucl Chem 304:95–105
Yu SJ, Mei HY, Chen X, Tan XL, Ahmad M, Alsaedi A, Hayat T, Wang XK (2015) Impact of environmental conditions on the sorption behavior of radionuclide 90Sr(II) on Na-montmorillonite. J Mol Liq 203:39–46
Zhao G, Jiang L, He Y, Li J, Dong H, Wang X, Hu W (2011) Sulfonated graphene for persistent aromatic pollutant management. Adv Mater 23:3959–3963
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Zhao GX, Wen T, Yang X, Yang SB, Liao JL, Hu J, Shao DD, Wang XK (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41:6182–6188
Sun YB, Wang Q, Chen CL, Tan XL, Wang XK (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027
Sun YB, Shao DD, Chen CL, Yang SB, Wang XK (2013) Highly efficient enrichment of radionuclides on graphene oxide supported polyaniline. Environ Sci Technol 47:9904–9910
Hui Q, Liu HT, Gao Y (2015) Removal of Sr(II) from aqueous solutions using polyacrylamide modified graphene oxide composites. J Mol Liq 208:394–401
Wang X, Yu JT (2015) Application of Fe3O4/graphene oxide composite for the separation of Cs(I) and Sr(II) from aqueous solution. J Radioanal Nucl Chem 303:807–813
Wen T, Wu XL, Liu MC, Xing ZH, Wang XK, Xu AW (2014) Efficient capture of strontium from aqueous solutions using graphene oxide-hydroxyapatite nanocomposites. Dalton Trans 43:7464–7472
Chen H, Shao D, Li J, Wang X (2014) The uptake of radionuclides from aqueous solution by poly(amidoxime) modified reduced graphene oxide. Chem Eng J 254:623–634
Romanchuk AY, Slesarev AS, Kalmykov SN, Kosynkin DV, Tour J (2013) Graphene oxide for effective radionuclide removal. Phys Chem Chem Phys 15:2321–2327
Yang S, Chen C, Chen Y, Li J, Wang D, Wang X, Hu W (2015) Competitive adsorption of Pb(II), Ni(II) and Sr(II) ions on graphene oxides: a combined experimental and theoretical study. ChemPlusChem 80:480–484
Tan XL, Wang XK, Geckeis H, Rabung Th (2008) Sorption of Eu(III) on humic acid or fulvic acid bound to alumina studied by SEM-EDS, XPS, TRLFS and batch techniques. Environ Sci Technol 42:6532–6537
Perdew JP, Burke K, Ernzerhof M (1997) Generalized gradient approximation made simple. Phys Rev Lett 78:1396
Balabanov NB, Peterson KA (2005) Systematically convergent basis sets for transition metals. I. All-electron correlation consistent basis sets for the 3d elements Sc-Zn. J Chem Phys 123:064107
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Frisch MJ, Trucks G, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani GE, Barone V, Mennucci B, Petersson GA (2009) Gaussian 09, revision A. 02. Gaussian, Inc., Wallingford
Lee S, Anderson PR, Bunker GB, Karanfil C (2004) EXAFS study of Zn sorption mechanisms on montmorillonite. Environ Sci Technol 38:5426–5432
Sheng GD, Yang ST, Sheng J, Hu J, Tan XL, Wang XK (2011) Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS and EXAFS techniques. Environ Sci Technol 45:7718–7726
Ho YS, Wase DAJ, Forster CF (1996) Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ Technol 17:71–77
Ijagbemi CO, Baek MH, Kim DS (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546
Mckay G, Poots VJP (1980) Kinetics and diffusion-process in color removal from effluent using wood as an adsorbent. J Chem Technol Biotechnol 30:279–292
Bhattacharyya KG, Gupta SS (2008) Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl Clay Sci 41:1–9
Weber WJ, Morris JC (1963) Kinetics of adsorption of carbon from solutions. J Sanit Eng Div Am Soc Civ Eng 89:31–63
Gupta SS, Bhattacharyya KG (2006) Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium deriviates. J Hazard Mater 128:247–257
Kowal-Fouchard A, Drot R, Simoni E, Ehrhardt JJ (2004) Use of spectroscopic techniques for uranium(VI)/montmorillonite interaction modeling. Environ Sci Technol 38:1399–1407
Sun YB, Li JX, Wang XK (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Abollino O, Giacomino A, Malandrino M, Mentasti E (2006) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38:227–236
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X (2015) Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ Sci Technol 49:4255–4262
Yang S, Ren X, Zhao G, Shi W, Montavon G, Grambow B, Wang X (2015) Competitive Sorption and selective sequence of Cu(II) and Ni(II) on montmorillonite: batch, modelling, EPR and XAFS studies. Geochim Cosmochim Acta 166:129–145
Barbier F, Duc G, Petit-Ramel M (2000) Adsorption of lead and cadmium ions from aqueous solution to the montmorillonite/water interface. Colloid Surf A Physicochem Eng Aspects 166:153–159
Shao D, Li J, Wang X (2014) Poly(amidoxime)-reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater. Sci China Chem 57:1449–1458
Mercer KL, Tobiason JE (2008) Removal of arsenic from high ionic strength solutions: effects of ionic strength, pH, and preformed versus in situ formed HFO. Environ Sci Technol 42:3797–3802
Reddad Z, Gerente C, Andres Y, Cloirec LP (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Song W, Shao D, Lu S, Wang X (2014) Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets. Sci China Chem 57:1291–1299
Ding CC, Cheng WC, Sun YB, Wang XK (2015) Effects of Bacillus subtilis on the reduction of U(VI) by nano-Fe0. Geochim Cosmochim Acta 165:86–107
Hayes KF, Leclie JO (1987) Modeling ionic strength effect on cation adsorption at hydrous oxide/solution interfaces. J Colloid Interface Sci 115:564–572
Yang SB, Hu J, Chen CL, Shao DD, Wang XK (2011) Mutual effect of Pb(II) and humic acid adsorption onto multiwalled carbon nanotubes/poly(acrylamide) composites from aqueous solution. Environ Sci Technol 45:3621–3627
Takahashi Y, Minai Y, Ambe H, Makide Y, Ambe F (1999) Comparison of adsorption behavior of multiple inorganic ions on kaolinite and silica in the presence of humic acid using the multitracer technique. Geochim Cosmochim Acta 63:815–836
Ozcan A, Oncu EM, Ozcan AS (2006) Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloid Surf A 277:90–97
Zhao Y, Shao ZY, Chen CL, Hu J, Chen HL (2014) Effect of environmental conditions on the adsorption behavior of Sr(II) by Na-rectorite. Appl Clay Sci 87:1–6
Wang XX, Lu SS, Chen L, Li JX, Dai SY, Wang XK (2015) Efficient removal of Eu(III) from aqueous solutions using super-adsorbent of bentonite-polyacrylamide composites. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4115-4
Tahir SS, Rauf N (2003) Thermodynamic studied of Ni(II) adsorption onto bentonite from aqueous solution. J Chem Thermodyn 35:2003–2009
Lan JH, Cao DP, Wang WC, Smit B (2010) Doping of alkali, alkaline-earth, and transition metals in covalent-organic frameworks for enhancing CO2 capture by first-principles calculations and molecular simulations. ACS Nano 4:4225–4237
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, under Grant No. 41-130-36-HiCi. The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, S., Wang, X., Dai, S. et al. Investigation of 90Sr(II) sorption onto graphene oxides studied by macroscopic experiments and theoretical calculations. J Radioanal Nucl Chem 308, 721–732 (2016). https://doi.org/10.1007/s10967-015-4425-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4425-6