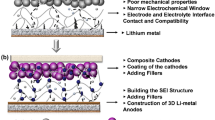
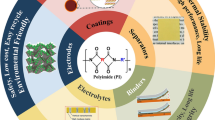
Abstract
Lithium-ion batteries (LIBs) exhibiting high capacity and energy density are in high demand in emerging markets such as electric vehicles and energy storage systems. However, these LIBs often show intrinsic shorter cycle life and higher risk of short circuit, which may result in thermal runaway and explosion. This work reviewed those polymers employed to improve cycling performance and safety of LIBs. First, some novel separator membranes, which prevent the direct contact of cathode with anode to induce disastrous short circuit, were developed with an aim at imparting safety to LIBs. For example, composite separators comprising ceramic and polymer show higher abuse tolerance for LIBs. Surface modification of the cathode active materials by polymers (e.g., polyimide (PI)) results in greatly enhanced electrochemical performance of LIBs, especially for layered LiNixCoyMnzO2. Another unique technology was developed, which involves coating reactive oligomer/polymer on the particle surface of cathode active materials to effectively limit the probability of short circuit and, eventually, thermal runaway of cells in an abusive environment. The fundamental mechanisms of the secondary oligomer/polymer reaction to form a dense highly cross-linked film on the pellet surface to inhibit thermal runaway were then discussed. This was followed by the last topic on polymer coated anodes used to suppress Li dendrite formation at the anode surface. Li dendrites may cause short circuit and capacity fading during cycling in LIBs. Some representative polymers including PI, polyurea and polycyanoacrylate that can greatly inhibit dendrite formation at anode surface were discussed.

















Similar content being viewed by others
References
Whittingham MS (1976) Electrical energy storage and intercalation chemistry. Science 192(4244):1126–1127
Mizushima K, Jones PC, Wiseman PJ, Goodenough JB (1981) LixCoO2 (0<x⩽1): A new cathode material for batteries of high energy density. Sol Stat Ion 3–4:171–174
Abraham KM (2015) Prospects and Limits of Energy Storage in Batteries. J Phys Chem Lett 6(5):830–844
Winter M, Barnett B, Xu K (2018) Before Li Ion Batteries. Chem Rev 118(23):11433–11456
Schmuch R, Wagner R, Hörpel G, Placke T et al (2018) Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 3(4):267–278
Bai Y, Wang X, Yang S, Zhang X et al (2012) The effects of FePO4-coating on high-voltage cycling stability and rate capability of Li[Ni0.5Co0.2Mn0.3]O2. J Alloys Compd 541:125–131
Betz J, Bieker G, Meister P, Placke T et al (2019) Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv Energy Mater 9(6):1803170
Chen Y, Zhang Y, Wang F, Wang Z et al (2014) Improve the structure and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material by nano-Al2O3 ultrasonic coating. J Alloys Compd 611:135–141
Hu G, Zhang M, Wu L, Peng Z et al (2017) Effects of Li2SiO3 coating on the performance of LiNi0.5Co0.2Mn0.3O2 cathode material for lithium ion batteries. J Alloys Compd 690:589–597
Lee S-W, Kim M-S, Jeong JH, Kim D-H et al (2017) Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J Power Sources 360:206–214
Liu X-H, Kou L-Q, Shi T, Liu K et al (2014) Excellent high rate capability and high voltage cycling stability of Y2O3-coated LiNi0.5Co0.2Mn0.3O2. J Power Sources 267:874–880
Liu L, Li M, Chu L, Jiang B et al (2020) Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog Mater Sci 111:100655
Qian Y, Niehoff P, Börner M, Grützke M et al (2016) Influence of electrolyte additives on the cathode electrolyte interphase (CEI) formation on LiNi1/3Mn1/3Co1/3O2 in half cells with Li metal counter electrode. J Power Sources 329:31–40
Xia Y, Zheng J, Wang C, Gu M (2018) Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 49:434–452
Evertz M, Horsthemke F, Kasnatscheew J, Börner M et al (2016) Unraveling transition metal dissolution of Li1.04Ni1/3Co1/3Mn1/3O2 (NCM 111) in lithium ion full cells by using the total reflection X-ray fluorescence technique. J Power Sources 329:364–371
Noh H-J, Youn S, Yoon CS, Sun Y-K (2013) Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J Power Sources 233:121-130
Lagadec MF, Zahn R, Wood V (2019) Characterization and performance evaluation of lithium-ion battery separators. Nat Energy 4(1):16–25
Lee H, Yanilmaz M, Toprakci O, Fu K et al (2014) A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ Sci 7(12):3857–3886
Ding L, Zhang D, Zhang S, Wu T et al (2021) Microporous structure and mechanical behavior of separators used for lithium-ion battery. J Polym Res 28(3):98
Xu G, Ding L, Wu T, Xiang M et al (2018) Effect of high molecular weight on pore formation and various properties of microporous membrane used for lithium-ion battery separator. J Polym Res 25(8):166
Sabetzadeh N, Gharehaghaji AA, Javanbakht M (2019) Porous PAN micro/nanofiber membranes with potential application as Lithium-ion battery separators: physical, morphological and thermal properties. J Polym Res 26(1):20
Chen C, Xu R, Chen X, Xie J et al (2016) Influence of cocrystallization behavior on structure and properties of HDPE/LLDPE microporous membrane. J Polym Res 23(3):46
Sakarya O, Kurama S, Gunkaya G (2016) Effect of Al2O3 Nanofiller on ion conductivity, transmittance, and glass transition temperature of PEI:LiTFSI:PC:EC polymer electrolytes. J Polym Res 24(1):14
Gogia A, Wang Y, Rai AK, Bhattacharya R et al (2021) Binder-Free, Thin-Film Ceramic-Coated Separators for Improved Safety of Lithium-Ion Batteries. ACS Omega 6(6):4204–4211
Takemura D, Aihara S, Hamano K, Kise M et al (2005) A powder particle size effect on ceramic powder based separator for lithium rechargeable battery. J Power Sources 146(1):779–783
Ding Y, Zhang P, Long Z, Jiang Y et al (2008) Preparation of PVdF-based electrospun membranes and their application as separators. Sci Technol Advan Mater 9(1):015005
Walkowiak M, Zalewska A, Jesionowski T, Pokora M (2007) Stability of poly(vinylidene fluoride-co-hexafluoropropylene)-based composite gel electrolytes with functionalized silicas. J Power Sources 173(2):721–728
Du Pasquier A, Warren PC, Culver D, Gozdz AS et al (2000) Plastic PVDF-HFP electrolyte laminates prepared by a phase-inversion process. Solid State Ionics 135(1):249–257
Kim KM, Park N-G, Ryu KS, Chang SH (2006) Characteristics of PVdF-HFP/TiO2 composite membrane electrolytes prepared by phase inversion and conventional casting methods. Electrochim Acta 51(26):5636–5644
Miao R, Liu B, Zhu Z, Liu Y et al (2008) PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries. J Power Sources 184(2):420–426
Subramania A, Kalyana Sundaram NT, Sathiya Priya AR, Vijaya Kumar G (2007) Preparation of a novel composite micro-porous polymer electrolyte membrane for high performance Li-ion battery. J Membr Sci 294(1):8–15
Lundquist JT, Lundsager CB, Palmer NI, Troffkin HJ (1985) Battery separator, in https://www.patentsgooglecom/patent/US4650730A/en, U. State, Editor. Celgard LLC: United State
Yu WC, Hux SE (1998) Method of making a trilayer battery separator, in https://www.patentsgooglecom/patent/US5952120A/en. 1998: United State
Callahan RW, Call RW, Harleson KJ, Yu TH (1999) Battery separators with reduced splitting propensity, in https://www.patentsgooglecom/patent/US6602593B1/en?oq=US6602593B1. Celgard LLC: United State
Jung B, Lee B, Jeong Y-C, Lee J et al (2019) Thermally stable non-aqueous ceramic-coated separators with enhanced nail penetration performance. J Power Sources 427:271–282
Arora P, Zhang Z (2004) Battery Separators. Chem Rev 104(10):4419–4462
Orendorff CJ (2012) The Role of Separators in Lithium-Ion Cell Safety. Interf Magaz 21(2):61–65
Friesen A, Horsthemke F, Mönnighoff X, Brunklaus G et al (2016) Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J Power Sources 334:1–11
Roth EP, Doughty DH, Pile DL (2007) Effects of separator breakdown on abuse response of 18650 Li-ion cells. J Power Sources 174(2):579–583
Zhang C, Li H, Wang S, Cao Y et al (2020) A polyethylene microsphere-coated separator with rapid thermal shutdown function for lithium-ion batteries. J Energy Chem 44:33–40
Zhang H, Zhou M-Y, Lin C-E, Zhu B-K (2015) Progress in polymeric separators for lithium ion batteries. RSC Adv 5(109):89848–89860
Choi J-A, Kim SH, Kim D-W (2010) Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J Power Sources 195(18):6192–6196
Jung YS, Cavanagh AS, Gedvilas L, Widjonarko NE et al (2012) Improved functionality of lithium-Ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes. Adv Energy Mater 2(8):1022–1027
Ould Ely T, Kamzabek D, Chakraborty D (2019) Batteries Safety: Recent Progress and Current Challenges. Front Ener Res 7:71
Zhang SS (2013) Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J Power Sources 231:153–162
Feng X, Ouyang M, Liu X, Lu L et al (2018) Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Materials 10:246–267
Wang Q, Ping P, Zhao X, Chu G et al (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Wu W, Wang S, Wu W, Chen K et al (2019) A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers Manage 182:262–281
Tian X, Yi Y, Fang B, Yang P et al (2020) Design Strategies of Safe Electrolytes for Preventing Thermal Runaway in Lithium Ion Batteries. Chem Mater 32(23):9821–9848
Wang X, Yasukawa E, Kasuya S (2001) Nonflammable Trimethyl Phosphate Solvent-Containing Electrolytes for Lithium-Ion Batteries: I. Fundamental Properties J Electrochem Soc 148(10):A1058
Zhang SS (2006) A review on electrolyte additives for lithium-ion batteries. J Power Sources 162(2):1379–1394
Tambio SJ, Roberge H, Xiong J, Soudan P et al (2021) Charge Transport Limitations to the Power Performance of LiNi0.5Mn0.3Co0.2O2 Composite Electrodes with Carbon Nanotubes. J Electrochem Soc 168(11):110508
Wang Q, Ping P, Sun J, Chen C (2010) Improved thermal stability of lithium ion battery by using cresyl diphenyl phosphate as an electrolyte additive. J Power Sources 195(21):7457–7461
Liu K, Liu W, Qiu Y, Kong B et al (2017) Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci Adv 3(1):e1601978
Shim E-G, Nam T-H, Kim J-G, Kim H-S et al (2007) Electrochemical performance of lithium-ion batteries with triphenylphosphate as a flame-retardant additive. J Power Sources 172(2):919–924
Xu B, Qian D, Wang Z, Meng YS (2012) Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng R Rep 73(5):51–65
Ryu H-H, Park K-J, Yoon CS, Sun Y-K (2018) Capacity fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation?. Chem Mater 30(3):1155–1163
Liu W, Oh P, Liu X, Lee M-J et al (2015) Nickel-Rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54(15):4440–4457
Chen Z, Chao D, Lin J, Shen Z (2017) Recent progress in surface coating of layered LiNixCoyMnzO2 for lithium-ion batteries. Mater Res Bull 96:491–502
Xin F, Zhou H, Zong Y, Zuba M et al (2021) What is the Role of Nb in Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries?. ACS Energy Lett 6(4):1377–1382
Yang Z, Lu J, Bian D, Zhang W et al (2014) Stepwise co-precipitation to synthesize LiNi1/3Co1/3Mn1/3O2 one-dimensional hierarchical structure for lithium ion batteries. J Power Sources 272:144–151
Zhou H, Xin F, Pei B, Whittingham MS (2019) What Limits the Capacity of Layered Oxide Cathodes in Lithium Batteries?. ACS Energy Lett 4(8):1902–1906
Bak S-M, Hu E, Zhou Y, Yu X et al (2014) Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl Mater Interfaces 6(24):22594–22601
Geng L, Liu J, Wood DL, Qin Y et al (2020) Probing Thermal Stability of Li-Ion Battery Ni-Rich Layered Oxide Cathodes by means of Operando Gas Analysis and Neutron Diffraction. ACS Appl Ener Mater 3(7):7058–7065
Cao G, Jin Z, Zhu J, Li Y et al (2020) A green Al2O3 metal oxide coating method for LiNi0.5Co0.2Mn0.3O2 cathode material to improve the high voltage performance. J Alloys Compd 832:153788
Zhang X, Belharouak I, Li L, Lei Y et al (2013) Structural and Electrochemical Study of Al2O3 and TiO2 Coated Li1.2Ni0.13Mn0.54Co0.13O2 Cathode Material Using ALD. Adv Ener Mater 3(10):1299–1307
Qiu Q, Huang X, Chen Y, Tan Y et al (2014) Al2O3 coated LiNi1/3Co1/3Mn1/3O2 cathode material by sol–gel method: Preparation and characterization. Ceram Int 40(7, Part B):10511–10516
Araki K, Taguchi N, Sakaebe H, Tatsumi K et al (2014) Electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathode material modified by coating with Al2O3 nanoparticles. J Power Sources 269:236–243
Zhao J, Aziz S, Wang Y (2014) Hierarchical functional layers on high-capacity lithium-excess cathodes for superior lithium ion batteries. J Power Sources 247:95–104
Liu W, Wang M, Xl G, Zhang W et al (2012) Improvement of the high-temperature, high-voltage cycling performance of LiNi0.5Co0.2Mn0.3O2 cathode with TiO2 coating. J Alloys Compd 543:181–188
Chen C, Tao T, Qi W, Zeng H et al (2017) High-performance lithium ion batteries using SiO2-coated LiNi0.5Co0.2Mn0.3O2 microspheres as cathodes. J Alloys Compd 709:708–716
Cho Y, Cho J (2010) Significant Improvement of LiNi[sub 0.8]Co[sub 0.15]Al[sub 0.05]O[sub 2] Cathodes at 60°C by SiO[sub 2] Dry Coating for Li-Ion Batteries. J Electrochem Soc 157(6):A625
Zhang X, Zhang P, Zeng T, Yu Z et al (2021) Improving the Structure Stability of LiNi0.8Co0.15Al0.05O2 by Double Modification of Tantalum Surface Coating and Doping. ACS Appl Ener Mater 4(8):8641–8652
Liang Y, Cai J, Liu D, Chen Z (2021) Surface modification of Nickel-Rich cathode materials by ionically conductive materials at room temperature. Energ Technol 9(10):2100422
Sun Y-K, Lee M-J, Yoon CS, Hassoun J et al (2012) The role of AlF3 coatings in improving electrochemical cycling of li-enriched nickel-manganese oxide electrodes for li-Ion batteries. Adv Mater 24(9):1192–1196
Kim AY, Strauss F, Bartsch T, Teo JH et al (2019) Stabilizing effect of a hybrid surface coating on a Ni-Rich NCM cathode material in all-solid-state batteries. Chem Mater 31(23):9664–9672
Park J-H, Cho J-H, Kim J-S, Shim E-G et al (2012) High-voltage cell performance and thermal stability of nanoarchitectured polyimide gel polymer electrolyte-coated LiCoO2 cathode materials. Electrochim Acta 86:346–351
Park J-H, Cho J-H, Lee E-H, Kim J-M et al (2013) Thickness-tunable polyimide nanoencapsulating layers and their influence on cell performance/thermal stability of high-voltage LiCoO2 cathode materials for lithium-ion batteries. J Power Sources 244:442–449
Park J-H, Cho J-H, Kim S-B, Kim W-S et al (2012) A novel ion-conductive protection skin based on polyimide gel polymer electrolyte: application to nanoscale coating layer of high voltage LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries. J Mater Chem 22(25):12574–12581
Zhang J, Lu Q, Fang J, Wang J et al (2014) Polyimide encapsulated lithium-rich cathode material for high voltage lithium-ion battery. ACS Appl Mater Interfaces 6(20):17965–17973
Park J-H, Kim J-S, Shim E-G, Park K-W et al (2010) Polyimide gel polymer electrolyte-nanoencapsulated LiCoO2 cathode materials for high-voltage Li-ion batteries. Electrochem Commun 12(8):1099–1102
Bordeneuve H, Guillemet-Fritsch S, Rousset A, Schuurman S et al (2009) Structure and electrical properties of single-phase cobalt manganese oxide spinels Mn3−xCoxO4 sintered classically and by spark plasma sintering (SPS). J Solid State Chem 182(2):396–401
Abouimrane A, Compton OC, Deng H, Belharouak I et al (2011) Improved Rate Capability in a High-Capacity Layered Cathode Material via Thermal Reduction. Electrochem Solid-State Lett 14(9):A126
Cho J-H, Park J-H, Lee M-H, Song H-K et al (2012) A polymer electrolyte-skinned active material strategy toward high-voltage lithium ion batteries: a polyimide-coated LiNi0.5Mn1.5O4 spinel cathode material case. Ener Environ Sci 5(5):7124–7131
Lu Q, Fang J, Yang J, Feng X et al (2014) A polyimide ion-conductive protection layer to suppress side reactions on Li4Ti5O12 electrodes at elevated temperature. RSC Adv 4(20):10280–10283
Abdelhamid ME, O’Mullane AP, Snook GA (2015) Storing energy in plastics: a review on conducting polymers & their role in electrochemical energy storage. RSC Adv 5(15):11611–11626
Rudge A, Davey J, Raistrick I, Gottesfeld S et al (1994) Conducting polymers as active materials in electrochemical capacitors. J Power Sources 47(1):89–107
Goto F, Abe K, Ikabayashi K, Yoshida T et al (1987) The polyaniline/lithium battery. J Power Sources 20(3):243–248
Meng Q, Cai K, Chen Y, Chen L (2017) Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 36:268–285
Posudievsky OY, Kozarenko OA, Dyadyun VS, Koshechko VG et al (2012) Electrochemical performance of mechanochemically prepared polyaniline doped with lithium salt. Synth Met 162(24):2206–2211
Su D, Zhang J, Dou S, Wang G (2015) Polypyrrole hollow nanospheres: stable cathode materials for sodium-ion batteries. Chem Commun 51(89):16092–16095
Li S, Shu K, Zhao C, Wang C et al (2014) One-step synthesis of graphene/polypyrrole nanofiber composites as cathode material for a biocompatible zinc/polymer battery. ACS Appl Mater Interfaces 6(19):16679–16686
Zhao F, Li Y, Feng W (2018) Recent advances in applying vulcanization/inverse vulcanization methods to achieve high-performance sulfur-containing polymer cathode materials for Li–S batteries. Small Meth 2(11):1800156
Oh SH, Lee CW, Chun DH, Jeon JD et al (2014) A metal-free and all-organic redox flow battery with polythiophene as the electroactive species. J Mater Chem A 2(47):19994–19998
Heeger AJ (2001) Semiconducting and metallic polymers: The fourth generation of polymeric materials. J Phys Chem B 105(36):8475–8491
Heeger AJ (2001) Semiconducting and metallic polymers: The fourth generation of polymeric materials (Nobel Lecture). Angew Chem Int Ed 40(14):2591–2611
Ghosh S, Maiyalagan T, Basu RN (2016) Nanostructured conducting polymers for energy applications: towards a sustainable platform. Nanoscale 8(13):6921–6947
Jia X, Ge Y, Shao L, Wang C et al (2019) Tunable conducting polymers: Toward sustainable and versatile batteries. ACS Sustain Chem Eng 7(17):14321–14340
Xiong X, Ding D, Wang Z, Huang B et al (2014) Surface modification of LiNi0.8Co0.1Mn0.1O2 with conducting polypyrrole. J Solid State Electrochem 18(9):2619–2624
Ju SH, Kang I-S, Lee Y-S, Shin W-K et al (2014) Improvement of the cycling performance of LiNi0.6Co0.2Mn0.2O2 cathode active materials by a dual-conductive polymer coating. ACS Appl Mater Interfac 6(4):2546–2552
Liu X, Li H, Li D, Ishida M et al (2013) PEDOT modified LiNi1/3Co1/3Mn1/3O2 with enhanced electrochemical performance for lithium ion batteries. J Power Sources 243:374–380
Lepage D, Michot C, Liang G, Gauthier M et al (2011) A soft chemistry approach to coating of LiFePO4 with a conducting polymer. Angew Chem Int Ed 50(30):6884–6887
Lee Y-S, Lee K-S, Sun Y-K, Lee YM et al (2011) Effect of an organic additive on the cycling performance and thermal stability of lithium-ion cells assembled with carbon anode and LiNi1/3Co1/3Mn1/3O2 cathode. J Power Sources 196(16):6997–7001
Wu F, Liu J, Li L, Zhang X et al (2016) Surface modification of Li-Rich cathode materials for lithium-ion batteries with a PEDOT:PSS conducting polymer. ACS Appl Mater Interfaces 8(35):23095–23104
Lee HD, Jung GJ, Lee HS, Kim T et al (2016) Improved stability of lithium-ion battery cathodes using conducting polymer binders. Sci Adv Mater 8(1):84–88
Ghosh S, Rasmusson J, Inganäs O (1998) Supramolecular self-assembly for enhanced conductivity in conjugated polymer blends: Ionic crosslinking in blends of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) and poly(vinylpyrrolidone). Adv Mater 10(14):1097–1099
Ko I-H, Kim S-J, Lim J, Yu S-H et al (2016) Effect of PEDOT:PSS coating on manganese oxide nanowires for lithium ion battery anodes. Electrochim Acta 187:340–347
Fedorková A, Oriňáková R, Oriňák A, Talian I et al (2010) PPy doped PEG conducting polymer films synthesized on LiFePO4 particles. J Power Sources 195(12):3907–3912
Fedorková A, Oriňáková R, Oriňák A, Wiemhöfer H-D et al (2010) Surface treatment of LiFePO4 cathode material with PPy/PEG conductive layer. J Solid State Electrochem 14(12):2173–2178
Hu D, Zhang Q, Tian J, Chen L et al (2021) High-temperature storage deterioration mechanism of cylindrical 21700-type batteries using Ni-Rich cathodes under different SOCs. ACS Appl Mater Interfaces 13(5):6286–6297
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264
Golubkov AW, Fuchs D, Wagner J, Wiltsche H et al (2014) Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv 4(7):3633–3642
Wang F-M, Lo S-C, Cheng C-S, Chen J-H et al (2011) Self-polymerized membrane derivative of branched additive for internal short protection of high safety lithium ion battery. J Membr Sci 368(1):165–170
Wang H, Du Z, Liu L, Zhang Z et al (2020) Study on the thermal runaway and its propagation of lithium-ion batteries under low pressure. Fire Technol 56(6):2427–2440
Doughty DH, Roth EP (2012) A General Discussion of Li Ion Battery Safety. Electrochem Soc Interface 21:37-44
Lin C-C, Wu H-C, Pan J-P, Su C-Y et al (2013) Investigation on suppressed thermal runaway of Li-ion battery by hyper-branched polymer coated on cathode. Electrochim Acta 101:11–17
Takamatsu D, Koyama Y, Orikasa Y, Mori S et al (2012) First In Situ Observation of the LiCoO2 Electrode/Electrolyte Interface by Total-Reflection X-ray Absorption Spectroscopy. Angew Chem Int Ed 51(46):11597–11601
Pham Q-T, Hsu J-M, Wang F-M, Huang X-C et al (2016) Kinetics of nucleation-controlled polymerization of N, N′-bismaleimide-4,4′- diphenylmethane/barbituric acid. Thermochim Acta 641:1–7
Yoon W-S, Kim K-B, Kim M-G, Lee M-K et al (2002) Oxygen Contribution on Li-Ion Intercalation−Deintercalation in LiCoO2 Investigated by O K-Edge and Co L-Edge X-ray Absorption Spectroscopy. J Phys Chem B 106(10):2526–2532
Kim YJ, Kim H, Kim B, Ahn D et al (2003) Electrochemical Stability of Thin-Film LiCoO2 Cathodes by Aluminum-Oxide Coating. Chem Mater 15(7):1505–1511
Pham Q-T, Zhan Y-X, Wang F-M, Chern C-S (2019) Mechanisms and kinetics of non-isothermal polymerization of N, N′-bismaleimide-4,4′-diphenylmethane with barbituric acid in dimethyl sulfoxide. Thermochim Acta 676:139–144
Pham Q-T, Hsu Y-J, Wang F-M, Chern C-S (2018) Mechanisms and kinetics of non-isothermal polymerization of N, N′-bismaleimide-4,4′-diphenylmethane with 2-thiobarbituric acid. Thermochim Acta 668:80–86
Pham Q-T, Hsu J-M, Shao W-J, Zhan Y-X et al (2017) Mechanisms and kinetics of non-isothermal polymerization of N, N′-bismaleimide-4,4′-diphenylmethane with 1,3-dimethylbarbituric acid. Thermochim Acta 658:31–37
Pham Q-T, Hsu J-M, Shao W-J, Wang F-M et al (2017) Mechanisms and kinetics of isothermal polymerization of N, N′-bismaleimide-4,4′-diphenylmethane with 5,5-dimethylbarbituric acid in the presence of triphenylphosphine. Thermochim Acta 655:234–241
Pham Q-T, Yu F-E, Hsu J-M, Chiang C-H et al (2016) Kinetics of polymerization of N, N′-bismaleimide-4,4′-diphenylmethane, barbituric acid and aminopropyl phenylsiloxane oligomer. J Taiwan Inst Chem Eng 67:88–97
Yu F-E, Lee H-Y, Hsu J-M, Pan J-P et al (2015) Effects of initial composition and temperature on the kinetics of polymerizations of N, N′-bismaleimide-4,4′-diphenylmethane with barbituric acid. J Taiwan Inst Chem Eng 52:181–190
Liu H-M, Saikia D, Wu H-C, Su C-Y et al (2014) Towards an understanding of the role of hyper-branched oligomers coated on cathodes, in the safety mechanism of lithium-ion batteries. RSC Adv 4(99):56147–56155
Wu Y-S, Pham Q-T, Yang C-C, Chern C-S et al (2021) Study of electrochemical performance and thermal property of LiNi0.5Co0.2Mn0.3O2 cathode materials coated with a novel oligomer additive for high-safety lithium-ion batteries. Chem Eng J 405:126727
Bailey JR, Hatfield MJ, Henke KR, Krepps MK et al (2001) Transition metal complexes of 2,4,6-trimercapto-1,3,5-triazine (TMT): potential precursors to nanoparticulate metal sulfides. J Organomet Chem 623(1):185–190
MacNeil DD, Dahn JR (2001) The Reaction of Charged Cathodes with Nonaqueous Solvents and Electrolytes: I. Li[sub 0.5]CoO[sub 2]. J Electrochem Soc 148(11):A1205
MacNeil DD, Hatchard TD, Dahn JR (2001) A comparison between the high temperature electrode /electrolyte reactions of Li[sub x]CoO[sub 2] and Li[sub x]Mn[sub 2]O[sub 4]. J Electrochem Soc 148(7):A663
Baba Y, Okada S, Yamaki J-i (2002) Thermal stability of LixCoO2 cathode for lithium ion battery. Solid State Ionics 148(3):311–316
Yu Y, Wang J, Zhang P, Zhao J (2017) A detailed thermal study of usual LiNi0.5Co0.2Mn0.3O2, LiMn2O4 and LiFePO4 cathode materials for lithium ion batteries. J Ener Stor 12:37–44
He Y (2005) Rapid thermal conductivity measurement with a hot disk sensor: Part 1. Theoretical considerations Thermochim Acta 436(1):122–129
Wang R, Cui W, Chu F, Wu F (2020) Lithium metal anodes: Present and future. J Energy Chem 48:145–159
Wu F, Maier J, Yu Y (2020) Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem Soc Rev 49(5):1569–1614
Ye H, Zhang Y, Yin Y-X, Cao F-F et al (2020) An outlook on low-volume-change lithium metal anodes for long-life batteries. ACS Cent Sci 6(5):661–671
Zhang SS (2018) Problem, status, and possible solutions for lithium metal anode of rechargeable batteries. ACS Appl Ene Mater 1(3):910–920
Kwon D-S, Kim HJ, Shim J (2021) Dendrite-suppressing polymer materials for safe rechargeable metal battery applications: From the electro-chemo-mechanical viewpoint of macromolecular design. Macromol Rapid Commun 42(16):2100279
Guo Y, Li H, Zhai T (2017) Reviving lithium-metal anodes for next-generation high-energy batteries. Adv Mater 29(29):1700007
Jin D, Park J, Ryou M-H, Lee YM (2020) Structure-controlled Li Metal electrodes for post-Li-Ion batteries: Recent progress and perspectives. Adv Mater Interfaces 7(8):1902113
Zhang H, Eshetu GG, Judez X, Li C et al (2018) Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: Progress and perspectives. Angew Chem Int Ed 57(46):15002–15027
Cao D, Sun X, Li Q, Natan A et al (2020) Lithium dendrite in all-solid-state batteries: Growth mechanisms, suppression strategies, and characterizations. Matter 3(1):57–94
Meyerson ML, Papa PE, Heller A, Mullins CB (2021) Recent developments in dendrite-free lithium-metal deposition through tailoring of micro- and nanoscale artificial coatings. ACS Nano 15(1):29–46
Ren W, Zheng Y, Cui Z, Tao Y et al (2021) Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Ener Stor Mater 35:157–168
Liu H, Cheng X-B, Huang J-Q, Yuan H et al (2020) Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett 5(3):833–843
Sun Y, Zhao Y, Wang J, Liang J et al (2019) A novel organic polyurea thin film for ultralong-life lithium-metal anodes via molecular-layer deposition. Adv Mater 31(4):1806541
Hu Z, Zhang S, Dong S, Li W et al (2017) Poly(ethyl α-cyanoacrylate)-based artificial solid electrolyte interphase layer for enhanced interface stability of Li Metal anodes. Chem Mater 29(11):4682–4689
Li N-W, Shi Y, Yin Y-X, Zeng X-X et al (2018) A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew Chem Int Ed 57(6):1505–1509
Li S, Fan L, Lu Y (2019) Rational design of robust-flexible protective layer for safe lithium metal battery. Ener Stor Mater 18:205–212
Wang X, Pan Z, Zhuang J, Li G et al (2019) Simultaneously regulating lithium ion flux and surface activity for dendrite-free lithium metal anodes. ACS Appl Mater Interfaces 11(5):5159–5167
Kang IS, Lee Y-S, Kim D-W (2013) Improved cycling stability of lithium electrodes in rechargeable lithium batteries. J Electrochem Soc 161(1):A53–A57
Xu W, Wang J, Ding F, Chen X et al (2014) Lithium metal anodes for rechargeable batteries. Energy Environ Sci 7(2):513–537
Li N-W, Yin Y-X, Yang C-P, Guo Y-G (2016) An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv Mater 28(9):1853–1858
Xiong S, Xie K, Diao Y, Hong X (2012) Properties of surface film on lithium anode with LiNO3 as lithium salt in electrolyte solution for lithium–sulfur batteries. Electrochim Acta 83:78–86
Zhang SS (2012) Effect of discharge cutoff voltage on reversibility of lithium/sulfur batteries with LiNO3-Contained electrolyte. J Electrochem Soc 159(7):A920–A923
Liang X, Wen Z, Liu Y, Wu M et al (2011) Improved cycling performances of lithium sulfur batteries with LiNO3-modified electrolyte. J Power Sources 196(22):9839–9843
Cheng X-B, Hou T-Z, Zhang R, Peng H-J et al (2016) Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv Mater 28(15):2888–2895
Acknowledgements
This work was supported by Ministry of Science and Technology (109-2221-E-011-086-; 109-2221-E-011-088- ) Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of financial or non-financial competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pham, QT., Chern, CS. Applications of polymers in lithium-ion batteries with enhanced safety and cycle life. J Polym Res 29, 124 (2022). https://doi.org/10.1007/s10965-022-02946-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02946-2