Abstract
A key goal of systems biology is the predictive mathematical description of gene regulatory circuits. Different approaches are used such as deterministic and stochastic models, models that describe cell growth and division explicitly or implicitly etc. Here we consider simple systems of unregulated (constitutive) gene expression and compare different mathematical descriptions systematically to obtain insight into the errors that are introduced by various common approximations such as describing cell growth and division by an effective protein degradation term. In particular, we show that the population average of protein content of a cell exhibits a subtle dependence on the dynamics of growth and division, the specific model for volume growth and the age structure of the population. Nevertheless, the error made by models with implicit cell growth and division is quite small. Furthermore, we compare various models that are partially stochastic to investigate the impact of different sources of (intrinsic) noise. This comparison indicates that different sources of noise (protein synthesis, partitioning in cell division) contribute comparable amounts of noise if protein synthesis is not or only weakly bursty. If protein synthesis is very bursty, the burstiness is the dominant noise source, independent of other details of the model. Finally, we discuss two sources of extrinsic noise: cell-to-cell variations in protein content due to cells being at different stages in the division cycles, which we show to be small (for the protein concentration and, surprisingly, also for the protein copy number per cell) and fluctuations in the growth rate, which can have a significant impact.





Similar content being viewed by others
Notes
The first term is replaced by \(\alpha\mathcal {P}(P-b,t)\times \varTheta (P-b)\), where Θ is the Heaviside function with Θ(P-b)=1 for P≥b and Θ(P-b)=0 for P<b.
For constant burst sizes, the values of b must be integers and that the result for a single-step protein synthesis is recovered for b=1, where every transcription event leads to the synthesis of exactly one protein molecule. With stochastic burst sizes, however, b can have non-integer values and the single-step process is recovered by taking the limit b→0, while keeping b×α m constant.
In eukaryotic systems, they are believed to mostly reflect different states of the chromatin structure.
References
Alon, U.: Biological networks: the tinkerer as an engineer. Science 301, 1866–1867 (2003)
Andrianantoandro, E., Basu, S., Karig, D.K., Weiss, R.: Synthetic biology: new engineering rules for an emerging discipline. Mol. Syst. Biol. 2, 2006.0028 (2006)
Berg, O.G.: A model for the statistical fluctuations of protein numbers in a microbial population. J. Theor. Biol. 71, 587–603 (1978)
Berg, O.G., Paulsson, J., Ehrenberg, M.: Fluctuations in repressor control: thermodynamic constraints on stochastic focusing. Biophys. J. 79, 2944–2953 (2000)
Bernstein, J.A., Khodursky, A.B., Lin, P., Lin-Chao, S., Cohen, S.N.: Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc. Natl. Acad. Sci. USA 99, 9697–9702 (2002)
Bintu, L., Buchler, N.E., Garcia, H.G., Gerland, U., Hwa, T., Kondev, J., Phillips, R.: Transcriptional regulation by the numbers: models. Curr. Opin. Genet. Dev. 15, 116–124 (2005)
Bremer, H., Dennis, P.P.: Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt, F.C. (ed.) Escherichia coli and Salmonella, 2nd edn., pp. 1553–1569. ASM Press, Washington (1996)
Brenner, N., Shokef, Y.: Nonequilibrium statistical mechanics of dividing cell populations. Phys. Rev. Lett. 99, 138102 (2007)
Cai, L., Friedman, N., Xie, X.S.: Stochastic protein expression in individual cells at the single molecule level. Nature 440, 358–362 (2006)
Cook, D.L., Gerber, A.N., Tapscott, S.J.: Modeling stochastic gene expression: implications for haploinsufficiency. Proc. Natl. Acad. Sci. USA 95, 15641–15646 (1998)
Cookson, N.A., Cookson, S.W., Tsimring, L.S., Hasty, J.: Cell cycle-dependent variations in protein concentration. Nucleic Acids Res. 38, 2676–2681 (2010)
Cooper, S., Helmstetter, C.E.: Chromosome replication and the division cycle of Escherichia coli B/r. J. Mol. Biol. 31, 519–540 (1968)
Cooper, S.: Distinguishing between linear and exponential cell growth during the division cycle: single-cell studies, cell-culture studies, and the object of cell-cycle research. Theor. Biol. Med. Model. 3, 10 (2006)
Csete, M.E., Doyle, J.C.: Reverse engineering of biological complexity. Science 295, 1664–1669 (2002)
Elowitz, M.B., Levine, A.J., Siggia, E.D., Swain, P.S.: Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002)
Golding, I., Paulsson, J., Zawilski, S.M., Cox, E.C.: Real-time kinetics of gene activity in individual bacteria. Cell 123, 1025–1036 (2005)
Guido, N.J., Wang, X., Adalsteinsson, D., McMillen, D., Hasty, J., Cantor, C.R., Elston, T.C., Collins, J.J.: A bottom-up approach to gene regulation. Nature 439, 856–860 (2006)
Guptasarma, P.: Does replication-induced transcription regulate synthesis of the myriad low copy number proteins of Escherichia coli? BioEssays 17, 987–997 (1995)
Hasty, J., Pradines, J., Dolnik, M., Collins, J.J.: Noise-based switches and amplifiers for gene expression. Proc. Natl. Acad. Sci. USA 97, 2075–2080 (2000)
Hasty, J., McMillen, D., Collins, J.J.: Engineered gene circuits. Nature 420, 224–230 (2002)
Kaern, M., Elston, T.C., Blake, W.J., Collins, J.J.: Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 6, 451–464 (2005)
Klumpp, S., Hwa, T.: Growth-rate-dependent partitioning of RNA polymerases in bacteria. Proc. Natl. Acad. Sci. USA 105, 20245–20250 (2008)
Klumpp, S., Hwa, T.: Stochasticity and traffic jams in the transcription of ribosomal RNA: intriguing role of termination and antitermination. Proc. Natl. Acad. Sci. USA 105, 18159–18164 (2008)
Klumpp, S., Zhang, Z., Hwa, T.: Growth rate-dependent global effects on gene expression in bacteria. Cell 139, 1366–1375 (2009)
Klumpp, S.: Pausing and backtracking in transcription under dense traffic conditions. J. Stat. Phys. 142, 1252–1267 (2011)
Klumpp, S.: Growth-rate dependence reveals design principles of plasmid copy number control. PLoS ONE 6, e20403 (2011)
Ko, M.S.: A stochastic model for gene induction. J. Theor. Biol. 153, 181–194 (1991)
Kwok, R.: Five hard truths for synthetic biology. Nature 463, 288–290 (2010)
McAdams, H.H., Arkin, A.: Stochastic mechanisms in gene expression. Proc. Natl. Acad. Sci. USA 94, 814–819 (1997)
Mitarai, N., Dodd, I.B., Crooks, M.T., Sneppen, K.: The generation of promoter-mediated transcriptional noise in bacteria. PLoS Comput. Biol. 4, e1000109 (2008)
Nath, K., Koch, A.L.: Protein degradation in Escherichia coli. I. Measurement of rapidly and slowly decaying components. J. Biol. Chem. 245, 2889–2900 (1970)
Ozbudak, E.M., Thattai, M., Kurtser, I., Grossman, A.D., van Oudenaarden, A.: Regulation of noise in the expression of a single gene. Nat. Genet. 31, 69–73 (2002)
Paulsson, J.: Summing up the noise in gene networks. Nature 427, 415–418 (2004)
Paulsson, J.: Models of stochastic gene expression. Phys. Life Rev. 2, 157–175 (2005)
Peccoud, J., Ycart, B.: Markovian modeling of gene-product synthesis. Theor. Popul. Biol. 48, 222–234 (1995)
Rao, C.V., Wolf, D.M., Arkin, A.P.: Control, exploitation and tolerance of intracellular noise. Nature 420, 231–237 (2002)
Reeh, S., Pedersen, S.: Post-translational modification of Escherichia coli ribosomal protein S6. Mol. Gen. Genet. 173, 183–187 (1979)
Scott, M., Hwa, T., Ingalls, B.: Deterministic characterization of stochastic genetic circuits. Proc. Natl. Acad. Sci. USA 104, 7402–7407 (2007)
Scott, M., Gunderson, C.W., Mateescu, E.M., Zhang, Z., Hwa, T.: Interdependence of cell growth and gene expression: origins and consequences. Science 330, 1099–1102 (2010)
Spudich, J.L., Koshland, D.E. Jr.: Non-genetic individuality: chance in the single cell. Nature 262, 467–471 (1976)
Swain, P.S., Elowitz, M.B., Siggia, E.D.: Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc. Natl. Acad. Sci. USA 99, 12795–12800 (2002)
Tan, C., Marguet, P., You, L.: Emergent bistability by a growth-modulating positive feedback circuit. Nat. Chem. Biol. 5, 842–848 (2009)
Taniguchi, Y., Choi, P.J., Li, G.W., Chen, H., Babu, M., Hearn, J., Emili, A., Xie, X.S.: Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329, 533–538 (2010)
Thattai, M., van Oudenaarden, A.: Intrinsic noise in gene regulatory networks. Proc. Natl. Acad. Sci. USA 98, 8614–8619 (2001)
Walczak, A.M., Sasai, M., Wolynes, P.G.: Self-consistent proteomic field theory of stochastic gene switches. Biophys. J. 88, 828–850 (2005)
Wall, M.E., Hlavacek, W.S., Savageau, M.A.: Design of gene circuits: lessons from bacteria. Nat. Rev. Genet. 5, 34–42 (2004)
Wang, P., Robert, L., Pelletier, J., Dang, W.L., Taddei, F., Wright, A., Jun, S.: Robust growth of Escherichia coli. Curr. Biol. 20, 1099–1103 (2010)
Yu, J., Xiao, J., Ren, X., Lao, K., Xie, X.S.: Probing gene expression in live cells, one protein molecule at a time. Science 311, 1600–1603 (2006)
Acknowledgements
The authors would like to thank Angelo Valleriani for stimulating discussions during the course of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
R.M. and V.B. contributed equally.
Appendix
Appendix
1.1 A.1 Typical Values of the Parameters
Estimates of typical parameter values in the model organism E. coli are summarized in Table 1. Most of these can, for example, be estimated from the data of Ref. [43]. A few of them require additional comments: (i) In E. coli proteins are typically stable, i.e. β p ≈0. So far, no complete survey of protein stability has been made, but the total cellular protein mass was found to be stable [31] and early proteomics studies (2d-gels) also indicated that almost all proteins covered by their approach were stable [37]. Nevertheless, some proteins are known to be unstable and, in these cases, β p can be of the order of 1 min-1. (ii) Genes are typically present as a single copy in the genome. This means that the gene copy number per cell is 1 before the gene is replicated and 2 after replication. Average gene copy numbers are between 1 and 2, except at fast growth with doubling times T<60 min, where rounds of DNA replication overlap and the gene copy numbers can be larger [7, 12]. (iii) The cell volume doubles over the division cycle and its average value depends on the growth conditions [7]. The value given in the table should be taken as an order or magnitude estimate.
1.2 A.2 Models with Stochastic Protein Synthesis and Stochastic Division
A general method for solving processes involving different rules of protein synthesis and cell division has been described in Ref. [8]. This method allows us in most of the cases to find averages and standard deviation of the protein number. We will describe the method briefly here following [8]. Let P n be the protein content in the n th generation immediately after the cell division. Let λ n be the amount of protein produced and accumulated till the cell division time in generation n and q n be the fraction of protein inherited by the daughter cell at the time cell division. Then one can write

The protein generation as well as division can be taken from some distributions. If these distributions admit finite moments then in the steady-state the distributions of λ and q become independent and hence one can write

From here one can get all the moments for P, in particular 〈P〉=〈λ〉. Let us consider an example where we add protein with rate α in between every two cell divisions and where the protein number is divided deterministically into half at every cell division after every T time. In this case the synthesis of protein follows a binomial distribution giving 〈λ〉=δλ 2=αT and the division fraction is given by a delta function δ(q-1/2) with 〈q〉=1/2 and 〈q 2〉-〈q〉2=0. Thus Eq. (18) gives 〈P〉=〈λ〉 and \(\langle P^{2} \rangle= \frac{1}{3}(2\langle\lambda\rangle^{2} + \langle\lambda^{2} \rangle)\). After some algebra one finds \(\eta^{2} = \frac{\langle P^{2} \rangle- \langle P \rangle^{2}}{\langle P^{2}\rangle} = \frac{1}{3\langle P_{0} \rangle} \) which is one of the cases discussed in the main text.
1.3 A.3 Distribution of Protein Number and Concentration Due to Variation over the Division Cycle
The distribution of the protein number discussed in Sect. 5.1 is obtained by inverting the time-dependence of the protein copy number, P(t) to obtain t(P) and a transformation of variables in the age distribution from t to P, which leads to

Specifically, for the constant age distribution that describes averages over a single lineage, this leads to \(\varPhi (P)=\frac{d}{dP} t(P)\). As a consequence, the result for an arbitrary age distribution can be rewritten as

i.e., the distribution of protein number in a single lineage weighted with the age distribution of the corresponding inverse.
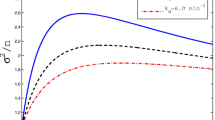
The distributions for the concentrations are obtained in an analogous fashion, but the calculation is technically more involved as the concentration is not a monotonic function of time (see, e.g. Fig. 1). We thus split the functions p lin(t) and p exp(t) into piecewise monotonic functions and determine the distributions for these separately. The concentration for linear cell growth, p lin(t), is monotonic in the intervals [0,t x ] and [t x ,T], and for p exp(t) we have three intervals [0,t x ], [t x ,t max] and [t max,T], where t max is the time where p exp(t) is maximal. The complete distributions Φ(p lin(t)) and Φ(p exp(t)) are then obtained by adding up the distributions from the respective intervals. The distributions for the concentrations Ψ(p), are again obtained for the corresponding intervals, weighted with the age distribution and summed up to yield the full distribution.
Rights and permissions
About this article
Cite this article
Marathe, R., Bierbaum, V., Gomez, D. et al. Deterministic and Stochastic Descriptions of Gene Expression Dynamics. J Stat Phys 148, 608–627 (2012). https://doi.org/10.1007/s10955-012-0459-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-012-0459-0