Abstract
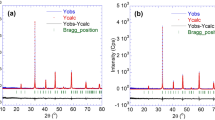
In this work, polycrystalline powder samples of Cu0.64Mn0.86[Fe(CN)6]·7.2H2O and Cu0.84Mn0.66[Fe(CN)6]·7.1 H2O have been synthesized by a coprecipitation method. The lattice parameter a is 10.053(8) Å and 10.083 Å for Cu0.64Mn0.86[Fe(CN)6]·7.2H2O and Cu0.84Mn0.66[Fe(CN)6]·7.1H2O, respectively. When the temperature reaches to 5 K, the magnetization is 7.262 emu/g and 0.142 emu/g for Cu0.64Mn0.86[Fe(CN)6]·7.2H2O and Cu0.84Mn0.66[Fe(CN)6]·7.1H2O, respectively. The coercive field and the remanent magnetization of Cu0.64Mn0.86[Fe(CN)6]·7.2H2O are 1204 Oe and 0.382 μB/fu, respectively. The coercive field and the remanent magnetization of Cu0.84Mn0.66[Fe(CN)6]·7.1H2O are 833 Oe and 0.681 μB/fu, respectively. In the initial magnetization curve, under the external magnetic field of 7 T, the magnetization of Cu0.64Mn0.86[Fe(CN)6]·7.2H2O and Cu0.84Mn0.66[Fe(CN)6]·7.1H2O is 2.13 μB/fu and 2.44 μB/fu, respectively. The difference between the calculated magnetic moment and the theoretical magnetic moment is quite large, which may be due to spin frustration.





Similar content being viewed by others
References
Sato, O., Iyoda, T., Fujishima, A., Hashimoto, K.: Photoinduced magnetization of a cobalt-iron cyanide. Science. 272, 704–705 (1996)
Bordage, A., Moulin, R., Fonda, E., Fornasieri, G., Rivière, E., Bleuzen, A.: Evidence of the core−shell structure of (photo)magnetic CoFe Prussian blue analogue nanoparticles and peculiar behavior of the surface secies. J. Am. Chem. Soc. 140, 10332–110342 (2015)
Adamson, J., Lucas, T.C., Cairns, A.B., Funnell, N.P., Tucker, M.G., Kleppe, A.K., Hriljac, J.A., Goodwin, A.L.: Competing hydrostatic compression mechanisms in nickel cyanide. Physica B. 479, 35–40 (2015)
Liu, M., Xu, M.: A coexistence of ferromagnetic and antiferromagnetic exchange interactions in Prussian blue analogue Cu0.47Ni0.48Mn0.55[Fe(CN)6]·XH2O. Inorg. Chem. Comm. 26(24–26), 24 (2012)
Phillips, A.E., Halder, G.L., Chapman, K.W., Goodwin, A.L., Kepert, C.J.: Zero thermal expansion in a flexible, stable framework: tetramethylammonium copper(I) zinc(II) cyanide. J. Am. Chem. Soc. 132, 10–11 (2009)
Adak, S., Daemen, L.L., Hartl, M., Williams, D., Summerhill, J., Nakotte, H.: Thermal expansion in 3d-metal Prussian blue analogs—a survey study. J. Solid State Chem. 184, 2854–2861 (2011)
Keskin, M., Ertas, M.: Dynamic magnetic hysteresis behaviors in a mixed spin(3/2, 2) bilayer system with different crystal-field interactions. J. Supercond. Nov. Magn. 30, 3439–3449 (2017)
Muthusamy, S., Charles, J., Renganathan, B., Sastikumar, D.: In situ growth of Prussian blue nanocubes on polypyrrole nanoparticles: facile synthesis, characterization and their application as fiber optic gas sensor. J. Mater. Sci. 53, 15401–15417 (2018)
Joseyphus, A.: Prussian blue modified Fe3O4 nanoparticles for Cs detoxification. J. Mater. Sci. 49, 7014–7022 (2014)
Wang, J., Zhuang, S., Liu, Y.: Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 374, 430–438 (2018)
Tokoro, H., Ohkoshi, S., Matsuda, T., Hozumi, T., Hashimoto, K.: Magnetic specific heat of the low-temperature phase of rubidium manganese hexacyanoferrate. Chem. Phys. Lett. 388, 379–383 (2006)
Vertelman, E.J.M., Maccallini, E., Gournis, D., Petra Rudolf, P., Bakas, T., Luzon, J., Broer, R., Pugzlys, A., Lummen, T.T.A., Loosdrecht, P.H.M., Koningsbruggen, P.J.: The influence of defects on the electron-transfer and magnetic properties of RbxMn[Fe(CN)6]y·ZH2O. Chem. Mater. 18(1953–1961), 1951 (2006)
Ohkoshi, S., Hashimoto, K.: Design of a novel magnet exhibiting photoinduced magnetic pole inversion based on molecular field theory. J. Am. Chem. Soc. 121, 10591–10597 (1999)
Yusuf, S.M., Kumar, A., Yakhmi, J.V.: Temperature- and magnetic-field-controlled magnetic pole reversal in a molecular magnetic compound. Appl. Phys. Lett. 95, 1825061–1825063 (2009)
Xia Y., Liu M., Li D. Hydrostatic pressure induced transformation of magnetism in a trimetallic CuMnFe Prussian blue analogue, J. Coord. Chem. 72, 491–491 (2019)
Lartigue, L., Oh, S., Prouzet, E., Guari, Y., Larionova, J.: Superspin-glass behavior of Co3[Fe(CN)6]2 Prussian blue nanoparticles confined in mesoporous silica. Mater. Chem. Phys. 132, 438–445 (2012)
Samain, L., Gilbert, B., Grandjean, F., Long, G.L., Strivay, D.: Redox reactions in Prussian blue containing paint layers as a result of light exposure. J. Anal. At. Spectrom. 28, 524–535 (2013)
Ohkoshi, S., Hashimoto, K.: Theoretical treatment of the mixed ferro-ferrimagnets composed of ternary-metal Prussian blue analogs in a paramagnetic. Phys. Rev. B. 60, 12820 (1999)
Ohkoshi, S., Iyoda, T., Fujishima, A., Hashimoto, K.: Magnetic properties of mixed ferro-ferrimagnets composed of Prussian blue analogs. Phys. Rev. B. 56, 11642–11652 (1997)
Konarev, D.V., Khasanov, S.S., Kuzmin, A.V., Otsuka, A., Yamochi, H., Saito, G., Lyubovskaya, R.N.: Effective magnetic coupling with strong spin frustration in (Ph3MeP+)(C60·-) and reversible C60·- dimerization in (Ph3MeP+)(C60·-)·C6H5CN. Effect of solvent on structure and properties. New J. Chem. 40, 2792–2798 (2016)
Liu, M., Xu, M.: Temperature dependent Jahn-Teller distortion in prussian blue analogue Cu0.47Ni0.48Mn0.55[Fe(CN)6]·7.8H2O. Z. Anorg. Allg. Chem. 639, 468–470 (2013)
Funding
This work was partially supported by National Natural Science Foundation of China (Grant No. 11447231), the National Undergraduate Innovation and Entrepreneurship Training Program Support Projects of China (Grant No. 201810555007), Research Foundation of Education Bureau of Hunan Province, China (Grant No. 14B151), and the Opening Project of Cooperative Innovation Center for Nuclear Fuel Cycle Technology and Equipment, University of South China (Grant No. 2019KFY10).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xia, Y., Zhang, G., Liu, M. et al. Magnetic Properties of Polycrystalline Compounds Cu0.64Mn0.86[Fe(CN)6]·7.2H2O and Cu0.84Mn0.66[Fe(CN)6]·7.1H2O. J Supercond Nov Magn 32, 3831–3835 (2019). https://doi.org/10.1007/s10948-019-05166-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-019-05166-w