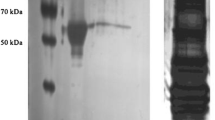
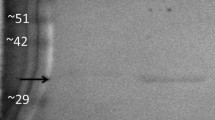
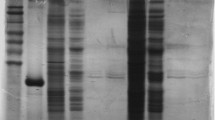
Abstract
The intertidal marine snail, Littorina littorea, has evolved to survive bouts of anoxia and extracellular freezing brought about by changing tides and subsequent exposure to harsh environmental conditions. Survival in these anoxic conditions depends on the animals entering a state of metabolic rate depression in order to maintain an appropriate energy production-consumption balance during periods of limited oxygen availability. This study investigated the kinetic, physical, and regulatory properties of pyruvate kinase (PK), which catalyzes the final reaction of aerobic glycolysis, from foot muscle of L. littorea to determine if the enzyme is differentially regulated in response to anoxia and freezing exposure. PK purified from foot muscle of anoxic animals exhibited a lower affinity for its substrate phosphoenolpyruvate than PK from control and frozen animals. PK from anoxic animals was also more sensitive to a number of allosteric regulators, including alanine and aspartate, which are key anaerobic metabolites in L. littorea. Furthermore, PK purified from anoxic and frozen animals exhibited greater stability compared to the non-stressed control animals, determined through high-temperature incubation studies. Phosphorylation of threonine and tyrosine residues was also assessed and demonstrated that levels of threonine phosphorylation of PK from anoxic animals were significantly higher than those of PK from control and frozen animals, suggesting a potential mechanism for regulating PK activity. Taken together, these results suggest that PK plays a role in suppressing metabolic rate in these animals during environmental anoxia exposure.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lutz PL, Storey KB (2011) Adaptations to variations in oxygen tension by vertebrates and invertebrates. Compr Physiol. https://doi.org/10.1002/cphy.cp130221
De Zwaan A, Putzer V (1985) Metabolic adaptations of intertidal invertebrates to environmental hypoxia (a comparison of environmental anoxia to exercise anoxia). Symp Soc Exp Biol 39:33–62
Aarset AV (1982) Freezing tolerance in intertidal invertebrates (a review). Comp Biochem Physiol -Part A Physiol. https://doi.org/10.1016/0300-9629(82)90264-X
English TE, Storey KB (2003) Freezing and anoxia stresses induce expression of metallothionein in the foot muscle and hepatopancreas of the marine gastropod Littorina littorea. J Exp Biol. https://doi.org/10.1242/jeb.00465
Murphy DJ (1983) Freezing Resistance in Intertidal Invertebrates. Annu Rev Physiol. https://doi.org/10.1146/annurev.ph.45.030183.001445
Larade K, Storey KB (2002) Reversible suppression of protein synthesis in concert with polysome disaggregation during anoxia exposure in Littorina littorea. Mol Cell Biochem. https://doi.org/10.1023/A:1014811017753
Larade K, Storey KB (2007) Arrest of transcription following anoxic exposure in a marine mollusc. Mol Cell Biochem. https://doi.org/10.1007/s11010-007-9468-8
Biggar KK, Kornfeld SF, Maistrovski Y, Storey KB (2012) MicroRNA regulation in extreme environments: differential expression of MicroRNAs in the intertidal snail Littorina littorea during extended periods of freezing and anoxia. Genomics Proteomics Bioinforma. https://doi.org/10.1016/j.gpb.2012.09.002
Storey KB, Lant B, Anozie OO, Storey JM (2013) Metabolic mechanisms for anoxia tolerance and freezing survival in the intertidal gastropod Littorina littorea. Comp Biochem Physiol. https://doi.org/10.1016/j.cbpa.2013.03.00
Krivoruchko A, Storey KB (2015) Turtle anoxia tolerance: biochemistry and gene regulation. Biochim Biophys Acta. https://doi.org/10.1016/j.bbagen.2015.02.001
Hand SC, Hardewig I (1996) Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol. https://doi.org/10.1146/annurev.ph.58.030196.002543
Brooks SPJ, Storey KB (1997) Glycolytic controls in estivation and anoxia: a comparison of metabolic arrest in land and marine molluscs. Comp Biochem Physiol 118:1103–1114
Russell EL, Storey KB (1995) Anoxia and freezing exposures stimulate covalent modification of enzymes of carbohydrate metabolism in Littorina littorea. J Comp Physiol B. https://doi.org/10.1007/BF00301477
Lama JL, Bell RAV, Storey KB (2013) Hexokinase regulation in the hepatopancreas and foot muscle of the anoxia-tolerant marine mollusk. Comp Biochem Physiol. https://doi.org/10.1016/j.cbpb.2013.07.001
Shahriari A, Dawson NJ, Bell RAV, Storey KB (2013) Stable suppression of lactate dehydrogenase activity during anoxia in the foot muscle of Littorina littorea and the potential role of acetylation as a novel posttranslational regulatory mechanism. Enzyme Res. https://doi.org/10.1155/2013/461374
Argaud D, Roth H, Wiernsperger N, Leverve XM (1993) Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur J Biochem. https://doi.org/10.1111/j.1432-1033.1993.tb17886.x
Rognstad R, Katz J (1977) Role of pyruvate kinase in the regulation of gluconeogenesis from L lactate. J Biol Chem 252:1831–1833
Plaxton WC, Storey KB (1984) Purification and properties of aerobic and anoxic forms of pyruvate kinase from red muscle tissue of the channelled whelk, Busycotypus canaliculatum. Eur J Biochem. https://doi.org/10.1111/j.1432-1033.1984.tb08367.x
Plaxton WC, Storey KB (1985) Purification and properties of aerobic and anoxic forms of pyruvate kinase from the hepatopancreas of the channelled whelk, Busycotypus canaliculatum. Arch Biochem Biophys. https://doi.org/10.1016/0003-9861(85)90788-X
Whitwam RE, Storey KS (1990) Pyruvate kinase from the land snail Otala lactea: regulation by reversible phosphorylation during estivation and anoxia. J Exp Biol 154:321–337
Smolinski MB, Mattice JJL, Storey KB (2017) Regulation of pyruvate kinase in skeletal muscle of the freeze tolerant wood frog, Rana sylvatica. Cryobiology. https://doi.org/10.1016/j.cryobiol.2017.06.002
Mattice AMS, MacLean IA, Childers CL, Storey KB (2018) Characterization of pyruvate kinase from the anoxia tolerant turtle, Trachemys scripta elegans: a potential role for enzyme methylation during metabolic rate depression. PeerJ. https://doi.org/10.7717/peerj.4918
Bell RAV, Storey KB (2018) Purification and characterization of skeletal muscle pyruvate kinase from the hibernating ground squirrel, Urocitellus richardsonii: potential regulation by posttranslational modification during torpor. Mol Cell Biochem. https://doi.org/10.1007/s11010-017-3192-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. https://doi.org/10.1016/0003-2697(76)90527-3
Brooks SPJ (1994) A program for analyzing enzyme rate data obtained from a microplate reader. Biotechniques 17:1154–1161
Brooks SPJ (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13:906–911
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal omega. Mol Syst Biol. https://doi.org/10.1038/msb.2011.75
Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. https://doi.org/10.1002/pmic.200300771
Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. https://doi.org/10.1093/nar/gkr1122
Storey KB, Storey JM (1990) Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q Rev Biol. https://doi.org/10.1086/416717
Storey KB (1997) Metabolic regulation in mammalian hibernation: Enzyme and protein adaptations. Comp Biochem Physiol. https://doi.org/10.1016/S0300-9629(97)00238-7
Cowan KJ, Storey KB (1999) Reversible phosphorylation control of skeletal muscle pyruvate kinase and phosphofructokinase during estivation in the spadefoot toad, Scaphiopus couchii. Mol Cell Biochem. https://doi.org/10.1023/A:1006932221288
Storey KB, Storey JM (2004) Oxygen limitation and metabolic rate depression. In: Storey KB (ed) Functional metabolism, 1st edn. Wiley, Hoboken, pp 415–442
Churchill TA, Storey KB (1996) Metabolic responses to freezing and anoxia by the periwinkle Littorina littorea. J Therm Biol. https://doi.org/10.1016/0306-4565(95)00022-4
Wieser W (1980) Metabolic end products in three species of marine gastropods. J Mar Biol Assoc UK. https://doi.org/10.1017/S0025315400024231
Storey KB (1986) Aspartate activation of pyruvate kinase in anoxia tolerant molluscs. Comp Biochem Physiol. https://doi.org/10.1016/0305-0491(86)90151-3
Sokolova IM, Pörtner HO (2001) Temperature effects on key metabolic enzymes in Littorina saxatilis and Littorina obtusata from different latitudes and shore levels. Mar Biol. https://doi.org/10.1007/s002270100557
Sokolova IM, Bock C, Pörtner HO (2000) Resistance to freshwater exposure in White Sea Littorina spp. I: anaerobic metabolism and energetics. J Comp Physiol. https://doi.org/10.1007/s003600050264
Hoyaux J, Gilles R, Jeuniaux C (1976) Osmoregulation in molluscs of the intertidal zone. Comp Biochem Physiol Part A. https://doi.org/10.1016/S0300-9629(76)80157-0
Taylor PM, Andrews EB (1988) Osmoregulation in the intertidal gastropod Littorina littorea. J Exp Mar Bio Ecol. https://doi.org/10.1016/0022-0981(88)90210-9
Pannunzio TM, Storey KB (1998) Antioxidant defenses and lipid peroxidation during anoxia stress and aerobic recovery in the marine gastropod Littorina littorea. J Exp Mar Bio Ecol. https://doi.org/10.1016/S0022-0981(97)00132-9
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev. https://doi.org/10.1152/physrev.1988.68.1.27
Abboud J, Storey KB (2013) Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ. https://doi.org/10.7717/peerj.12
Titanji VPK, Zetterqvist Ö, Engström L (1976) Regulation in vitro of rat liver pyruvate kinase by phosphorylation-dephosphorylation reactions, catalyzed by cyclic-AMP dependent protein kinases and a histone phosphatase. Biochim Biophys Acta. https://doi.org/10.1016/0005-2744(76)90011-5
Ljungström O, Berglund L, Engström L (1976) Studies on the kinetic effects of adenosine-3′: 5′-monophosphatedependent phosphorylation of purified pig-liver pyruvate kinase type L. Eur J Biochem. https://doi.org/10.1111/j.1432-1033.1976.tb10837.x
Acknowledgements
This study was funded through a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (#6793). K.B.S. holds the Canada Research Chair in Molecular Physiology. M.B.S was funded through an Ontario Graduate Scholarship. The authors thank J.M. Storey for editorial review of this manuscript.
Funding
This study was funded through a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (#6793). K.B.S. holds the Canada Research Chair in Molecular Physiology. M.B.S was funded through an Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
MBS carried out experiments, analyzed the data, and helped draft the manuscript. AV analyzed the data and drafted the manuscript. SRG carried out experiments and revised the manuscript. KBS secured funding, conceived the experiments, and helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Protocols were approved by the Carleton University Animal Care Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smolinski, M.B., Varma, A., Green, S.R. et al. Purification and Regulation of Pyruvate Kinase from the Foot Muscle of the Anoxia and Freeze Tolerant Marine Snail, Littorina littorea. Protein J 39, 531–541 (2020). https://doi.org/10.1007/s10930-020-09934-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09934-9