Abstract
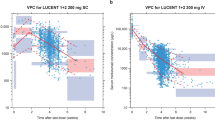
Efmarodocokin alfa (IL-22Fc) is a fusion protein of human IL-22 linked to the crystallizable fragment (Fc) of human IgG4. It has been tested in multiple indications including inflammatory bowel disease (IBD). The purposes of the present analyses were to describe the population pharmacokinetics (PK) of efmarodocokin alfa and perform pharmacodynamic (PD) analysis on the longitudinal changes of the PD biomarker REG3A after efmarodocokin alfa treatment as well as identify covariates that affect efmarodocokin alfa PK and REG3A PD. The data used for this analysis included 182 subjects treated with efmarodocokin alfa in two clinical studies. The population PK and PD analyses were conducted sequentially. Efmarodocokin alfa concentration–time data were analyzed using a nonlinear mixed-effects modeling approach, and an indirect response model was adopted to describe the REG3A PD data with efmarodocokin alfa serum concentration linked to the increase in REG3A. The analysis software used were NONMEM and R. A 3-compartment model with linear elimination best described the PK of efmarodocokin alfa. The estimated population-typical value for clearance (CL) was 1.12 L/day, and volume of central compartment was 6.15 L. Efmarodocokin alfa CL increased with higher baseline body weight, C-reactive protein, and CL was 27.6% higher in IBD patients compared to healthy subjects. The indirect response PD model adequately described the longitudinal changes of REG3A after efmarodocokin alfa treatment. A popPK and PD model for efmarodocokin alfa and REG3A was developed and covariates affecting the PK and PD were identified.



Similar content being viewed by others
Data sharing statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
References
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275(40):31335–31339. https://doi.org/10.1074/jbc.M005304200
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21(2):241–254. https://doi.org/10.1016/j.immuni.2004.07.007
Stefanich EG, Rae J, Sukumaran S, Lutman J, Lekkerkerker A, Ouyang W, Wang X, Lee D, Danilenko DM, Diehl L, Loyet KM, Herman A (2018) Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases. Biochem Pharmacol 152:224–235. https://doi.org/10.1016/j.bcp.2018.03.031
Rae J, Hackney J, Huang K, Keir M, Herman A (2021) Identification of an IL-22-dependent gene signature as a pharmacodynamic biomarker. Int J Mol Sci. https://doi.org/10.3390/ijms22158205
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14(3):282–289. https://doi.org/10.1038/nm1720
Chang JT (2020) Pathophysiology of inflammatory bowel diseases. N Engl J Med 383(27):2652–2664. https://doi.org/10.1056/NEJMra2002697
Rothenberg ME, Wang Y, Lekkerkerker A, Danilenko DM, Maciuca R, Erickson R, Herman A, Stefanich E, Lu TT (2019) Randomized phase I healthy volunteer study of UTTR1147A (IL-22Fc): a potential therapy for epithelial injury. Clin Pharmacol Ther 105(1):177–189. https://doi.org/10.1002/cpt.1164
Steel DM, Whitehead AS (1994) The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today 15(2):81–88. https://doi.org/10.1016/0167-5699(94)90138-4
Schultz DR, Arnold PI (1990) Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum 20(3):129–147. https://doi.org/10.1016/0049-0172(90)90055-k
van Beelen GA, Ostvik AE, Brenna O, Torp SH, Gustafsson BI, Sandvik AK (2013) REG gene expression in inflamed and healthy colon mucosa explored by in situ hybridisation. Cell Tissue Res 352(3):639–646. https://doi.org/10.1007/s00441-013-1592-z
Wagner F, Mansfield J, Lekkerkerker AN, Wang Y, Keir M, Dash A, Butcher B, Harder B, Orozco LD, Mar JS, Chen H, Rothenberg ME. A dose escalation randomization study of efmarodocokin alfa in healthy volunteers and patients with ulcerative colitis. Gut (Epub ahead of print)
Sperinde G, Liu L, Xu K, Bentley T, Sukumaran S, Lutman J, Huang C, Williams K, Hokom M, Fischer SK (2022) Challenges with development of a pharmacokinetics assay to measure a variably glycosylated fusion protein. Bioanalysis 14(1):7–18. https://doi.org/10.4155/bio-2021-0186
Martin JC, Beriou G, Heslan M, Bossard C, Jarry A, Abidi A, Hulin P, Menoret S, Thinard R, Anegon I, Jacqueline C, Lardeux B, Halary F, Renauld JC, Bourreille A, Josien R (2016) IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol 9(2):539–549. https://doi.org/10.1038/mi.2015.83
Pelczar P, Witkowski M, Perez LG, Kempski J, Hammel AG, Brockmann L, Kleinschmidt D, Wende S, Haueis C, Bedke T, Witkowski M, Krasemann S, Steurer S, Booth CJ, Busch P, Konig A, Rauch U, Benten D, Izbicki JR, Rosch T, Lohse AW, Strowig T, Gagliani N, Flavell RA, Huber S (2016) A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science 354(6310):358–362. https://doi.org/10.1126/science.aah5903
CTEP. Specific instructions for the use of protocol templates for organ dysfunction studies. National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/docs/CTEP_Organ_Dysfunction_Protocol_Template.docx. Accessed 19 Dec 2022
Ordas I, Mould DR, Feagan BG, Sandborn WJ (2012) Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 91(4):635–646. https://doi.org/10.1038/clpt.2011.328
Kapel N, Meillet D, Favennec L, Magne D, Raichvarg D, Gobert JG (1992) Evaluation of intestinal clearance and faecal excretion of alpha 1-antiproteinase and immunoglobulins during Crohn’s disease and ulcerative colitis. Eur J Clin Chem Clin Biochem 30(4):197–202. https://doi.org/10.1515/cclm.1992.30.4.197
Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, Mathot RA, Ponsioen CY, Lowenberg M, D’Haens GR (2015) Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 149(2):350-355 e352. https://doi.org/10.1053/j.gastro.2015.04.016
Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, Dijkmans BA, Aarden L (2005) Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 64(5):704–707. https://doi.org/10.1136/ard.2004.030452
Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, Davis HM, Zhou H (2009) Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 65(12):1211–1228. https://doi.org/10.1007/s00228-009-0718-4
Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM (2010) Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 48(5):297–308. https://doi.org/10.5414/cpp48297
Khan N, Patel D, Shah Y, Trivedi C, Yang YX (2017) Albumin as a prognostic marker for ulcerative colitis. World J Gastroenterol 23(45):8008–8016. https://doi.org/10.3748/wjg.v23.i45.8008
Vermeire S, Van Assche G, Rutgeerts P (2004) C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 10(5):661–665. https://doi.org/10.1097/00054725-200409000-00026
Dumoutier L, Lejeune D, Colau D, Renauld JC (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol 166(12):7090–7095. https://doi.org/10.4049/jimmunol.166.12.7090
Ogawa H, Fukushima K, Naito H, Funayama Y, Unno M, Takahashi K, Kitayama T, Matsuno S, Ohtani H, Takasawa S, Okamoto H, Sasaki I (2003) Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis 9(3):162–170. https://doi.org/10.1097/00054725-200305000-00003
Choi JH, Lee MY, Kim Y, Shim JY, Han SM, Lee KA, Choi YK, Jeon HM, Baek KH (2010) Isolation of genes involved in pancreas regeneration by subtractive hybridization. Biol Chem 391(9):1019–1029. https://doi.org/10.1515/BC.2010.101
Marafini I, Di Sabatino A, Zorzi F, Monteleone I, Sedda S, Cupi ML, Antenucci C, Biancheri P, Giuffrida P, Di Stefano M, Corazza GR, Pallone F, Monteleone G (2014) Serum regenerating islet-derived 3-alpha is a biomarker of mucosal enteropathies. Aliment Pharmacol Ther 40(8):974–981. https://doi.org/10.1111/apt.12920
Mukherjee S, Partch CL, Lehotzky RE, Whitham CV, Chu H, Bevins CL, Gardner KH, Hooper LV (2009) Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem 284(8):4881–4888. https://doi.org/10.1074/jbc.M808077200
Loonen LM, Stolte EH, Jaklofsky MT, Meijerink M, Dekker J, van Baarlen P, Wells JM (2014) REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol 7(4):939–947. https://doi.org/10.1038/mi.2013.109
Darnaud M, Dos Santos A, Gonzalez P, Augui S, Lacoste C, Desterke C, De Hertogh G, Valentino E, Braun E, Zheng J, Boisgard R, Neut C, Dubuquoy L, Chiappini F, Samuel D, Lepage P, Guerrieri F, Dore J, Brechot C, Moniaux N, Faivre J (2018) Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology 154(4):1009-1023 e1014. https://doi.org/10.1053/j.gastro.2017.11.003
Acknowledgements
This study was sponsored by Genentech, Inc. The authors would like to thank the patients who participated in this study, their families, the study coordinators, and the support staff at the clinical sites.
Funding
This study was sponsored by Genentech Inc.
Author information
Authors and Affiliations
Contributions
YY and AB conducted the analysis; YY and AL wrote the main manuscript; and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests. YY, MR, HD, BH, RZ, RO, NK, and AL are employees of Genentech Inc. and own stock in Roche Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Rothenberg, M.E., Ding, H.T. et al. Population pharmacokinetics and pharmacodynamics of efmarodocokin alfa (IL-22Fc). J Pharmacokinet Pharmacodyn 51, 141–153 (2024). https://doi.org/10.1007/s10928-023-09888-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-023-09888-2