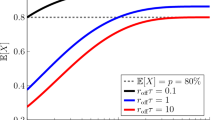
Abstract
Nonadherence to medication is a major public health problem. To combat nonadherence, some clinicians have suggested using “forgiving” drugs, which maintain efficacy in spite of delayed or missed doses. What pharmacokinetic (PK) and pharmacodynamic (PD) factors make a drug forgiving? In this paper, we address this question by analyzing a linear PK/PD model for a patient with imperfect adherence. We assume that the drug effect is far from maximal and consider direct effect, effect compartment (biophase), and indirect response PD models. We prove that the average drug effect relative to the clinically desired effect is simply the fraction of prescribed doses actually taken by the patient. Hence, under these assumptions, drug forgiveness cannot be defined in terms of the average effect. We argue that forgiveness should instead be understood in terms of effect fluctuations. We prove that the rates of PK absorption, PK elimination, and PD elimination are exactly equivalent for determining effect fluctuations. We prove all the aforementioned results for any pattern of nonadherence, including late doses, missed doses, drug holidays, extra doses, etc. To obtain quantitative estimates of effect fluctuations, we consider a simple statistical pattern of nonadherence and analytically calculate the coefficient of variation of effect. We further show how effect fluctuations can be reduced by taking an extra “make up” dose following a missed dose if any one of the aforementioned PK/PD rates is sufficiently slow. We illustrate some of our results for a nonlinear indirect response model of metformin.







Similar content being viewed by others
References
Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P et al (2012) A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73(5):691–705
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353(5):487–497
Eduardo S et al (2003) Adherence to long-term therapies: evidence for action. World Health Organization, Geneva
Brian HR, Pauline MH, Amit G, Patty M (2002) Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000011
Urquhart J (1998) Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv Drug Deliv Rev 33(3):207–219
Assawasuwannakit P, Braund R, Duffull SB (2015) Quantification of the forgiveness of drugs to imperfect adherence. CPT: Pharmacomet Syst Pharmacol 4(3):204–211
Assawasuwannakit P, Braund R, Duffull SB (2016) A framework for quantifying the influence of adherence and dose individualization. Clin Pharmacol Ther 99(4):452–459
Pellock JM, Brittain ST (2016) Use of computer simulations to test the concept of dose forgiveness in the era of extended-release (XR) drugs. Epilepsy Behav 55:21–23
Morrison A, Stauffer ME, Kaufman AS (2017) Relationship between adherence rate threshold and drug ‘forgiveness’. Clin Pharmacokinet 56(12):1435–1440
Véronique D (2011) Drug forgiveness and interpatient pharmacokinetic variability in tuberculosis. J Infect Dis 204:1827–1829
Lowy A, Munk VC, Ong SH, Burnier M, Vrijens B, Tousset EP, Urquhart J (2011) Effects on blood pressure and cardiovascular risk of variations in patients? adherence to prescribed antihypertensive drugs: role of duration of drug action. Int J Clin Pract 65(1):41–53
Nony P, Boissel J-P (2002) Use of sensitivity functions to characterise and compare the forgiveness of drugs. Clin Pharmacokinet 41(5):371–380
Gohore DG, Fenneteau F, Barrière O, Li J, Nekka F et al (2010) Rational drug delineation: a global sensitivity approach based on therapeutic tolerability to deviations in execution. Pharmacol Pharm 1(02):42
Osterberg LG, Urquhart J, Blaschke TF (2010) Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther 88(4):457–459
Boissel J-P, Nony P (2002) Using pharmacokinetic-pharmacodynamic relationships to predict the effect of poor compliance. Clin Pharmacokinet 41(1):1–6
Melanie AF, Marilyn EM, Donald EM (2012) Mechanism-based pharmacodynamic modeling. Computational toxicology. Springer, New York, pp 583–600
Hong Y, Rohatagi S, Habtemariam B, Walker JR, Schwartz SL, Mager DE (2008) Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol 48(6):696–707
Bender CM, Orszag SA (2013) Advanced mathematical methods for scientists and engineers I: Asymptotic methods and perturbation theory. Springer, New York
Counterman ED, Lawley SD (2021) What should patients do if they miss a dose of medication? A theoretical approach. J Pharmacokinet Pharmacodynam
Counterman ED, Lawley SD (2022) Designing drug regimens that mitigate nonadherence. Bull Math Biol 84(1):1–36
Stauffer ME, Hutson P, Kaufman AS, Morrison A (2017) The adherence rate threshold is drug specific. Drugs in R&D 17(4):645–653
Garnett WR, McLean AM, Zhang Y, Clausen S, Tulloch SJ (2003) Simulation of the effect of patient nonadherence on plasma concentrations of carbamazepine from twice-daily extended-release capsules. Curr Med Res Opin 19(6):519–525
Reed RC, Dutta S (2004) Predicted serum valproic acid concentrations in patients missing and replacing a dose of extended-release divalproex sodium. Am J Health Syst Pharm 61(21):2284–2289
Dutta S, Reed RC (2006) Effect of delayed and/or missed enteric-coated divalproex doses on valproic acid concentrations: simulation and dose replacement recommendations for the clinician 1. J Clin Pharm Ther 31(4):321–329
Ding J, Zhang Y, Jiao Z, Wang Y (2012) The effect of poor compliance on the pharmacokinetics of carbamazepine and its epoxide metabolite using monte carlo simulation. Acta Pharmacol Sin 33(11):1431–1440
Chen C, Wright J, Gidal B, Messenheimer J (2013) Assessing impact of real-world dosing irregularities with lamotrigine extended-release and immediate-release formulations by pharmacokinetic simulation. Ther Drug Monit 35(2):188–193
Gidal Barry E, Oneeb M, Jim F, Ziad H, Haichen Y, Jin Z, Randi F, Antonio L (2014) The practical impact of altered dosing on perampanel plasma concentrations: pharmacokinetic modeling from clinical studies. Epilepsy Behav 35:6–12
Brittain ST, Wheless JW (2015) Pharmacokinetic simulations of topiramate plasma concentrations following dosing irregularities with extended-release vs immediate-release formulations. Epilepsy Behav 52:31-36D
Sunkaraneni S, Blum D, Ludwig E, Chudasama V, Fiedler-Kelly J, Marvanova M, Bainbridge J, Phillips L (2018) Population pharmacokinetic evaluation and missed-dose simulations for eslicarbazepine acetate monotherapy in patients with partial-onset seizures. Clin Pharmacol Drug Dev 7(3):287–297
Hard ML, Wehr AY, Sadler BM, Mills RJ, Moltke L (2018) Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet 43(4):461–469
Elkomy MH (2020) Changing the drug delivery system: Does it add to non-compliance ramifications control? A simulation study on the pharmacokinetics and pharmacodynamics of atypical antipsychotic drug. Pharmaceutics 12(4):297
Jia-qin G, Guo Y, Jiao Z, Ding J, Li G-F (2020) How to handle delayed or missed doses: a population pharmacokinetic perspective. Eur J Drug Metab Pharmacokinet 45(2):163–172
Li J, Nekka F (2007) A pharmacokinetic formalism explicitly integrating the patient drug compliance. J Pharmacokinet Pharmacodyn 34(1):115–139
Lévy-Véhel P-E, Lévy-Véhel J (2013) Variability and singularity arising from poor compliance in a pharmacokinetic model I: the multi-IV case. J Pharmacokinet Pharmacodyn 40(1):15–39
Fermín LJ, Lévy-Véhel J (2017) Variability and singularity arising from poor compliance in a pharmacokinetic model II: the multi-oral case. J Math Biol 74(4):809–841
Jie M (2017) Stochastic modeling of random drug taking processes and the use of singular perturbation methods in pharmacokinetics. Ph.D. Thesis, The University of Utah
Hof F, Bridge LJ (2021) Exact solutions and equi-dosing regimen regions for multi-dose pharmacokinetics models with transit compartments. J Pharmacokinet Pharmacodyn 48(1):99–131
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34(5):711–726
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336(7653):1114–1117
Hammer SM, Vaida F, Bennett KK, Holohan MK, Sheiner L, Eron JJ, Wheat LJ, Mitsuyasu RT, Gulick RM, Valentine FT et al (2002) Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA 288(2):169–180
Sun J, Nagaraj HN, Reynolds NR (2007) Discrete stochastic models for compliance analysis based on an AIDS clinical trial group (ACTG) study. Biom J 49(5):731–741
Crauel H (2001) Random point attractors versus random set attractors. J Lond Math Soc (2) 63(2):413–427
Mattingly JC (1999) Ergodicity of \(2\)D Navier–Stokes equations with random forcing and large viscosity. Commun Math Phys 206(2):273–288
Schmalfuß B (1996) A random fixed point theorem based on Lyapunov exponents. Random Comput Dynam 4(4):257–268
Lawley SD, Mattingly JC, Reed MC (2015) Stochastic switching in infinite dimensions with applications to random parabolic PDE. SIAM J Math Anal 47(4):3035–3063
Lawley SD, Keener JP (2019) Electrodiffusive flux through a stochastically gated ion channel. SIAM J Appl Math 79(2):551–571
Norris JR (1998) Markov chains. Statistical & probabilistic mathematics. Cambridge University Press, Cambridge
Durrett R (2010) Probability: theory and examples, 4th edn. Cambridge University Press, Cambridge
Funding
SDL was supported by the National Science Foundation (Grant Nos. DMS-1944574 and DMS-1814832).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
In this appendix, we present the mathematical proofs and derivations of some results in the main text.
Proof of formula for E(t) in (21)
To verify (21), note first that (21) and the definitions in (22) imply that \(E(0)=0\), as desired. Next, the definition of E(t) in (18) and the ODE for \(c_{\text {e}}\) in (6) imply that E(t) satisfies
Differentiating (21) with respect to t and using the value of \(c_{ \text {p}}\) in (4) shows that (21) indeed satisfies (49), which verifies (21).□
Proof that \(\langle E \rangle =\mu \langle E^{\mathrm{perf}} \rangle\)
To prove \(\langle E \rangle =\mu \langle E^{\mathrm{perf}} \rangle\), we let \(k>0\) and consider the large T behavior of
Interchanging the sum and integral yields
By (30), the first term in (50) has the large T limit,
We claim that the second term in (50) vanishes as \(T\rightarrow \infty\). To see this, let \(\varepsilon \in (0,1)\) and observe that
Since (30) implies that
it follows that
To estimate the upper bound in (52), let \(\varepsilon _{2}>0\) and observe that (30) implies that if T is sufficiently large, then
and
Since \(\varepsilon \in (0,1)\) and \(\varepsilon _{2}>0\) are arbitrary, we conclude from (52) that
and therefore (50) and (51) imply
Finally, using the expression for E(t) in (25), the values of \(f_{n}\) and \(t_{n}\) in (26) for perfect adherence, and the result in (55), we conclude that
□
Derivation of \(\text {CV}(E^{\text {single}})\)
Using the model of nonadherence introduced in “Coefficient of variation estimate”, applying (33) gives
where the coefficients \(a_{ \text {p}0}\), \(a_{ \text {a} \text {p}}\), and \(a_{\text {e}0}\) are defined in (34), \({\lfloor }{t/\tau }{\rfloor }\) denotes the largest integer less than or equal to \(t/\tau\), and \(\{f_{n}\}_{n\in \mathbb {Z}}\) are as in (37). Note that we now take the sequence \(\{f_{n}\}_{n\in \mathbb {Z}}\) to be bi-infinite (i.e. \(n\in \mathbb {Z}\)), which is convenient for the analysis below.
For \(t\in [0,\tau ]\), define
where
Note that \(A_{ij}\) converges almost surely by the Weierstrass M-test since \(|f_{n}|\) is bounded by a deterministic constant for all \(n\in \mathbb {Z}\) (namely, \(|f_{n}|\le 1\) in this case). Random variables of the form in (57) are often referred to as random pullback attractors [42,43,44,45,46].
It then follows from (32) and Theorem 1 in [20] that
where \(\mathbb {E}\) denotes mathematical expectation. Using the definition of \(\Psi (t)\) in (56), equation (58) implies that \(\text {CV}(E^{\text {single}})\) requires calculating expectations of the form
These expectations are obtained immediately from Corollary 3 in [20]. In particular, we have that
Plugging these formulas into (58) and performing the integration yields for the formula for \(\text {CV}(E^{\text {single}})\) in (39).
Derivation of \(\mu ^{\text {double}}\)
For the double dose protocol in ““Make up” doses reduce variation for slow PK or PD”, it is immediate that \(\{f_{n}\}_{n\in \mathbb {Z}}\) is an irreducible, time-homogeneous Markov chain with the following transition probabilities,
Using standard Markov chain results (for example, see section 1.7 in [47]), a quick calculation shows that the stationary distribution of \(\{f_{n}\}_{n\in \mathbb {Z}}\) is
Using (30), Birkhoff’s ergodic theorem (for example, see section 7.2 in [48]) thus implies that the long-term fraction of doses taken for the double dose protocol is
Derivation of \(\text {CV}(E^{\text {double}})\)
The derivation of the formula for \(\text {CV}(E^{\text {double}})\) in (47) is identical to the derivation presented in “Derivation of \(\text {CV}(E^{\text {single}})\)” above except the formulas for the expectations in (59) are given by the formulas for the double dose protocol in Corollary 3 in [20].
Maximum plasma concentration for double dose protocol
Theorem 5 in [20] yields the following upper bound for the maximum possible plasma compartment concentration obtained by following the double dose protocol,
where \(\varphi\) is the following dimensionless factor,
and \(\langle c_{ \text {p}}^{\mathrm{perf}} \rangle =\frac{DF}{V}\frac{1}{\tau }\frac{1}{k_{ \text {p}0}}\) is the long-term average plasma concentration for perfect adherence. The upper bound in (60) is valid for any time \(t\ge 0\) and any sequence of remembering or forgetting \(\{\xi _{n}\}_{n\ge 0}\) for the adherence model in ““Make up” doses reduce variation for slow PK or PD” above. The first term in the righthand side of (60) is the maximum plasma concentration obtained by a patient with perfect adherence. The second term in the righthand side of (60) bounds the maximum possible increase in plasma concentration caused by the double dose protocol.
In Fig. 8, we plot the factor \(\varphi\) in (61). This plot shows that \(\varphi \ll 1\) if \(k_{ \text {a} \text {p}}\tau \ll 1\) and/or \(k_{ \text {p}0}\tau \ll 1\). This means that if the PK absorption rate is slow compared to the dosing interval (\(k_{ \text {a} \text {p}}\tau \ll 1\)) and/or the PK elimination rate is slow compared to the dosing interval (\(k_{ \text {p}0}\tau \ll 1\)), then the double dose protocol can at most cause the plasma concentration to rise only slightly above the concentration for perfect adherence.
Rights and permissions
About this article
Cite this article
McAllister, N.P., Lawley, S.D. A pharmacokinetic and pharmacodynamic analysis of drug forgiveness. J Pharmacokinet Pharmacodyn 49, 363–379 (2022). https://doi.org/10.1007/s10928-022-09808-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-022-09808-w