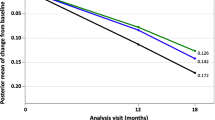
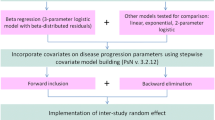
Abstract
Our objective was to develop a beta regression (BR) model to describe the longitudinal progression of the 11 item Alzheimer’s disease (AD) assessment scale cognitive subscale (ADAS-cog) in AD patients in both natural history and randomized clinical trial settings, utilizing both individual patient and summary level literature data. Patient data from the coalition against major diseases database (3,223 patients), the Alzheimer’s disease neruroimaging initiative study database (186 patients), and summary data from 73 literature references (representing 17,235 patients) were fit to a BR drug-disease-trial model. Treatment effects for currently available acetyl cholinesterase inhibitors, longitudinal changes in disease severity, dropout rate, placebo effect, and factors influencing these parameters were estimated in the model. Based on predictive checks and external validation, an adequate BR meta-analysis model for ADAS-cog using both summary-level and patient-level data was developed. Baseline ADAS-cog was estimated from baseline MMSE score. Disease progression was dependent on time, ApoE4 status, age, and gender. Study drop out was a function of time, baseline age, and baseline MMSE. The use of the BR constrained simulations to the 0–70 range of the ADAS-cog, even when residuals were incorporated. The model allows for simultaneous fitting of summary and patient level data, allowing for integration of all information available. A further advantage of the BR model is that it constrains values to the range of the original instrument for simulation purposes, in contrast to methodologies that provide appropriate constraints only for conditional expectations.









Similar content being viewed by others
References
Agresti A (1990) Categorical data analysis. Wiley, New York
Ashford JW, Schmitt FA (2001) Modeling the time-course of alzheimer dementia. Curr Psychiatry Rep 3(1):20–28
Ferrari S, Cribari-Neto F (2004) Beta regression for modelling rates and proportions. J Appl Stat 31(7):799–815
Gastonguay MR, French JL, Heitjan DF, Rogers JA, Ahn JE, Ravva P (2010) Missing data in model-based pharmacometric applications: points to consider. J Clin Pharmacol 50 (Suppl 1):63S
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis. Chapman & Hall/CRC, New York
Gelman A, Van MI, Verbeke G, Heitjan DF, Meulders M (2005) Multiple imputation for model checking: completed-data plots with missing and latent data. Biometrics 61(1):74–85
Gillespie WR, Rogers JA, Ito K, Gastonguay MR (2009) Population dose-response model for adas-cog scores in patients with alzheimers disease by meta-analysis of a mixture of summary and individual data. In: American Conference on Pharmacometrics. Mashantucket
Holford NH, Peace KE (1992) Methodologic aspects of a population pharmacodynamic model for cognitive effects in alzheimer patients treated with tacrine. Proc Natl Acad Sci 89(23): 11,466–11,470 http://www.pnas.org/content/89/23/11466.abstract
Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T (2010) Alzheimer’s disease working group: disease progression meta-analysis model in alzheimer’s disease. Alzheimers Dement 6(1):39–53. doi:10.1016/j.jalz.2009.05.665
Ito K, Corrigan B, Zhao Q, French J, Miller R, Soares H, Katz E, Nicholas T, Billing B, Anziano R, Fullerton T (2011) Alzheimer’s disease neuroimaging initiative: disease progression model for cognitive deterioration from alzheimer’s disease neuroimaging initiative database. Alzheimers Dement 7(2):151–160. doi:10.1016/j.jalz.2010.03.018
Kieschnick R, McCullough BD (2003) Regression analysis of variates observed on (0, 1): percentages, proportions and fractions. Stat Model 3:192–213
Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR (2005) How vague is vague? a simulation study of the impact of the use of vague prior distributions in mcmc using winbugs. Stat Med 24(15):2401–2428. doi:10.1002/sim.2112
L’Ecuyer P, Simard R, Chen EJ, Kelton WD (2001) An object-oriented random-number package with many long streams and substreams. Oper Res 50:1073–1075
Little RJA, Rubin DB (2002) Statistical analysis with missing data, 2nd edn. Wiley, New York
Ludden TM, Beal SL, Sheiner LB (1994) Comparison of the akaike information criterion, the schwarz criterion and the f test as guides to model selection. J Pharmacokinet Biopharm 22(5):431–445
Lunn D, Thomas A, Best N, Spiegelhalter D (2000) Winbugs—a bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
Mohs RC, Rosen WG, Davis KL (1983) The alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull 19(3):448–450
Olkin I, Tate RF (1961) Multivariate correlation models with mixed discrete and continuous variables. Ann Math Stat 32(2):448–465
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. ISBN: 3-900051-07-0
Romero K, Corrigan B, Neville J, Kopko S, Cantillon M (2011) Striving for an integrated drug-development process for neurodegeneration: the coalition against major diseases. Neurodegener Dis Manag 1(5):379–385
Romero K, de Mars M, Frank D, Anthony M, Neville J, Kirby L, Smith K, Woosley RL (2009) The coalition against major diseases: developing tools for an integrated drug development process for Alzheimer’s and Parkinson’s diseases. Clin Pharmacol Ther 86(4):365–367. doi:10.1038/clpt.2009.165
Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for alzheimer’s disease. Am J Psychiatry 141(11):1356–1364
Samtani MN, Farnum M, Lobanov V, Yang E, Raghavan N, DiBernardo A, Narayan V (2012) Alzheimer’s disease neuroimaging initiative: an improved model for disease progression in patients from the Alzheimer’s disease neuroimaging initiative. J Clin Pharmacol 52(5): 629–644
Stukel TA (1988) Generalized logistic models. J Am Stat Assoc 83(42):426–431
Tsoularis A, Wallace J (2002) Analysis of logistic growth models. Math Biosci 179(1):21–55
Williams-Faltaos D, Ying C, Wang Y, Gobburu J, Zhu H, Quantification of disease progression and drop-out for Alzheimer’s disease. Technical report, Food and Drug Administration
Acknowledgements
Critical Path Institutes Coalition Against Major Diseases is supported by grant No. U01FD003865 from the United States Food and Drug Administration and by Science Foundation Arizona under Grant No. SRG 0335-08. Data collection and sharing for the ADNI component of our combined data set was funded by the AD Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimers Association; Alzheimers Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is conducted for the Coalition Against Major Diseases and the Alzheimer’s Disease Neuroimaging Initiative.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. Data used in the preparation of this article were obtained from the Coalition Against Major Diseases database (CAMD).
Appendix: Linear approximation to the logit transformation
Appendix: Linear approximation to the logit transformation
One may obtain linear approximations to the logit and inverse logit transformations using first-order Taylor expansion of logit(x) around x = 0.5, resulting in the approximation \({\rm logit}(\mathit{ADAS}/70) \approx -2 + 4 \times (\mathit{ADAS}/70)\) , or for the inverse relationship. This linear approximation is not invoked for modeling per se, although the justification of our “operational likelihood” for summary statistics does invoke the existence of a linear approximation. Rather, its primary utility is to enable an approximate interpretation of model parameters on the original scale, this being important for both prior specification and model (posterior) summary.
We tabulate the approximate transformations used for each parameter in Table 7. With the exception of the population average intercept, all conversions rely on the linear approximation described above (in the case of the population average intercept, one may apply the inverse logit transformation directly, since this is a location parameter; similar exact transformations are not possible for scale parameters). Where appropriate, a multiplicative factor of 52 is also used to convert weekly rates to annual rates.
Rights and permissions
About this article
Cite this article
Rogers, J.A., Polhamus, D., Gillespie, W.R. et al. Combining patient-level and summary-level data for Alzheimer’s disease modeling and simulation: a beta regression meta-analysis. J Pharmacokinet Pharmacodyn 39, 479–498 (2012). https://doi.org/10.1007/s10928-012-9263-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9263-3