Abstract
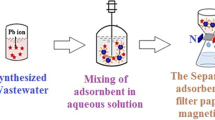
The problems associated with heavy metal pollutants in household and industrial wastewater affect both humans and the environment. Studies showed that bentonite/Fe3O4 nanoparticles and sodium alginate could be used for effective adsorption of lead ions. To this end, this study first synthesized four groups of bentonite magnetic nanoparticles (at the weights of 0.2, 0.4, 1, and 2 g of bentonite) and then examined their structural properties using the XRD, FT-IR and FE-SEM methods. In the next stage, the prepared magnetic nanoparticles were immobilized on alginate and the beads prepared for lead ions elimination in the synthetic pollutant solution at different adsorbent dosages, pH values, stirring rates, pollutant concentrations, and adsorption durations. The results show that the optimum condition for removal of Pb2+ was using 0.1 g of beads in solution pH of 7, contact time of 8 h for adsorption and stirring rate of 100 rpm. Furthermore, adsorption efficiency of the beads for adsorption of 30 ppm of Pb2+ solution was achieved about 98% and the adsorption of Pb2+ by using alginate magnetic beads following Freundlich isotherm model.
Graphic Abstract














Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Kubilay Ş, Gürkan R, Savran A, Şahan T (2007) Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 13:41–51. https://doi.org/10.1007/s10450-007-9003-y
Li R, Zhang T, Zhong H et al (2020) Bioadsorbents from algae residues for heavy metal ions adsorption: chemical modification, adsorption behaviour and mechanism. Environ Technol. https://doi.org/10.1080/09593330.2020.1723711
Ye S, Zeng G, Wu H et al (2019) The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour Conserv Recycl 140:278–285. https://doi.org/10.1016/j.resconrec.2018.10.004
Mandal S, Pu S, Adhikari S et al (2020) Progress and future prospects in biochar composites: application and reflection in the soil environment. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2020.1713030
Shi J, Pang J, Liu Q et al (2020) Simultaneous removal of multiple heavy metals from soil by washing with citric acid and ferric chloride. RSC Adv 10:7432–7442. https://doi.org/10.1039/C9RA09999A
Zheng X, Wu K, Sun P et al (2021) Effects of substrate types on the transformation of heavy metal speciation and bioavailability in an anaerobic digestion system. J Environ Sci 101:361–372. https://doi.org/10.1016/j.jes.2020.08.032
Lawal AA, Hassan MA, Ahmad Farid MA et al (2020) One-step steam pyrolysis for the production of mesoporous biochar from oil palm frond to effectively remove phenol in facultatively treated palm oil mill effluent. Environ Technol Innov 18:100730. https://doi.org/10.1016/j.eti.2020.100730
Hou F-L, Lv G-H, Teng D-X (2020) Spatial variability characteristics and environmental effects of heavy metals in surface riparian soils and surface sediments of Qinggeda Lake. Hum Ecol Risk Assess Int J 26:2027–2043. https://doi.org/10.1080/10807039.2019.1641790
Bereket G, Arog AZ, Özel MZ (1997) Removal of Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by adsorption on bentonite. J Colloid Interface Sci 187:338–343. https://doi.org/10.1006/jcis.1996.4537
Al-Rub FAA, El-Naas MH, Benyahia F, Ashour I (2004) Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem 39:1767–1773. https://doi.org/10.1016/j.procbio.2003.08.002
Verma R, Asthana A, Singh AK et al (2017) Novel glycine-functionalized magnetic nanoparticles entrapped calcium alginate beads for effective removal of lead. Microchem J 130:168–178. https://doi.org/10.1016/j.microc.2016.08.006
Katircioğlu H, Aslim B, Rehber Türker A et al (2008) Removal of cadmium(II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour Technol 99:4185–4191. https://doi.org/10.1016/j.biortech.2007.08.068
Peng L, Qin P, Lei M et al (2012) Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J Hazard Mater 209–210:193–198. https://doi.org/10.1016/j.jhazmat.2012.01.011
Nasrullah A, Bhat AH, Naeem A et al (2018) High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int J Biol Macromol 107:1792–1799. https://doi.org/10.1016/j.ijbiomac.2017.10.045
Bouberka Z, Kacha S, Kameche M et al (2005) Sorption study of an acid dye from an aqueous solutions using modified clays. J Hazard Mater 119:117–124. https://doi.org/10.1016/j.jhazmat.2004.11.026
Ait Sidhoum D, Socías-Viciana MM, Ureña-Amate MD et al (2013) Removal of paraquat from water by an Algerian bentonite. Appl Clay Sci 83–84:441–448. https://doi.org/10.1016/j.clay.2013.07.007
Dewangan T, Tiwari A, Bajpai AK (2009) Removal of arsenic ions from aqueous solutions by adsorption onto biopolymeric crosslinked calcium alginate beads. Toxicol Environ Chem 91:1055–1067. https://doi.org/10.1080/02772240802585012
Clark DE, Green HC (1936) Alginic acid and process of making same
Babu AN, Sree TR, Reddy DS et al (2019) Experimental and statistical analysis of As(III) adsorption from contaminated water using activated red mud doped calcium-alginate beads. Environ Technol. https://doi.org/10.1080/09593330.2019.1681520
Mahmoodi NM (2013) Magnetic ferrite nanoparticle–alginate composite: synthesis, characterization and binary system dye removal. J Taiwan Inst Chem Eng 44:322–330. https://doi.org/10.1016/j.jtice.2012.11.014
Naga Babu A, Krishna Mohan GV, Kalpana K, Ravindhranath K (2017) Removal of lead from water using calcium alginate beads doped with hydrazine sulphate-activated red mud as adsorbent. J Anal Methods Chem 2017:4650594. https://doi.org/10.1155/2017/4650594
Ravikumar KVG, Kubendiran H, Ramesh K et al (2020) Batch and column study on tetracycline removal using green synthesized NiFe nanoparticles immobilized alginate beads. Environ Technol Innov 17:100520. https://doi.org/10.1016/j.eti.2019.100520
Tan WS, Ting ASY (2014) Alginate-immobilized bentonite clay: adsorption efficacy and reusability for Cu(II) removal from aqueous solution. Bioresour Technol 160:115–118. https://doi.org/10.1016/j.biortech.2013.12.056
Hu Z-H, Omer AM, Ouyang X, Yu D (2018) Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int J Biol Macromol 108:149–157. https://doi.org/10.1016/j.ijbiomac.2017.11.171
Kumar R, Kim S-J, Kim K-H et al (2018) Removal of hazardous hexavalent chromium from aqueous phase using zirconium oxide-immobilized alginate beads. Appl Geochem 88:113–121. https://doi.org/10.1016/j.apgeochem.2017.04.002
Papageorgiou SK, Katsaros FK, Kouvelos EP et al (2006) Heavy metal sorption by calcium alginate beads from Laminaria digitata. J Hazard Mater 137:1765–1772. https://doi.org/10.1016/j.jhazmat.2006.05.017
Wu D, Gao Y, Li W et al (2016) Selective Adsorption of La3+ using a tough alginate-clay-poly(n-isopropylacrylamide) hydrogel with hierarchical pores and reversible re-deswelling/swelling cycles. ACS Sustain Chem Eng 4:6732–6743. https://doi.org/10.1021/acssuschemeng.6b01691
Gopalakannan V, Viswanathan N (2016) One pot synthesis of metal ion anchored alginate–gelatin binary biocomposite for efficient Cr(VI) removal. Int J Biol Macromol 83:450–459. https://doi.org/10.1016/j.ijbiomac.2015.10.010
Zhang H, Omer AM, Hu Z et al (2019) Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu(II) adsorption. Int J Biol Macromol 135:490–500. https://doi.org/10.1016/j.ijbiomac.2019.05.185
Ahmad T, Danish M, Kale P et al (2019) Optimization of process variables for biodiesel production by transesterification of flaxseed oil and produced biodiesel characterizations. Renew Energy 139:1272–1280. https://doi.org/10.1016/j.renene.2019.03.036
Abdelkrim S, Mokhtar A, Djelad A et al (2020) Chitosan/Ag-bentonite nanocomposites: preparation, characterization, swelling and biological properties. J Inorg Organomet Polym Mater 30:831–840. https://doi.org/10.1007/s10904-019-01219-8
Martínez-Costa JI, Maldonado Rubio MI, Leyva-Ramos R (2020) Degradation of emerging contaminants diclofenac, sulfamethoxazole, trimethoprim and carbamazepine by bentonite and vermiculite at a pilot solar compound parabolic collector. Catal Today 341:26–36. https://doi.org/10.1016/j.cattod.2018.07.021
Khan SA, Siddiqui MF, Khan TA (2020) Ultrasonic-assisted synthesis of polyacrylamide/bentonite hydrogel nanocomposite for the sequestration of lead and cadmium from aqueous phase: equilibrium, kinetics and thermodynamic studies. Ultrason Sonochem 60:104761. https://doi.org/10.1016/j.ultsonch.2019.104761
Belaroui LS, Millet JMM, Bengueddach A (2004) Characterization of lalithe, a new bentonite-type Algerian clay, for intercalation and catalysts preparation. Catal Today 89:279–286. https://doi.org/10.1016/j.cattod.2003.12.020
Oladipo AA, Gazi M (2014) Enhanced removal of crystal violet by low cost alginate/acid activated bentonite composite beads: Optimization and modelling using non-linear regression technique. J Water Process Eng 2:43–52. https://doi.org/10.1016/j.jwpe.2014.04.007
Yan L, Li S, Yu H et al (2016) Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol 301:632–640. https://doi.org/10.1016/j.powtec.2016.06.051
Removal of Cd and Pb Ions from Aqueous Solutions Using Bentonite-Modified Magnetic Nanoparticles | Semantic Scholar. https://www.semanticscholar.org/paper/Removal-of-Cd-and-Pb-Ions-from-Aqueous-Solutions-Al-Farhan/3c6c795ebd99620478bccccd665355aa2e87e3ce. Accessed 18 Mar 2020
Shi L, Zhang X, Chen Z (2011) Removal of Chromium(VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45:886–892. https://doi.org/10.1016/j.watres.2010.09.025
Shahwan T, Üzüm Ç, Eroğlu AE, Lieberwirth I (2010) Synthesis and characterization of bentonite/iron nanoparticles and their application as adsorbent of cobalt ions. Appl Clay Sci 47:257–262. https://doi.org/10.1016/j.clay.2009.10.019
Merrikhpour H, Jalali M (2013) Sorption processes of natural iranian bentonite exchanged with Cd2+, Cu2+, Ni2+, and Pb2+ cations. Chem Eng Commun 200:1645–1665. https://doi.org/10.1080/00986445.2012.759561
Çelekli A, Yavuzatmaca M, Bozkurt H (2010) An eco-friendly process: predictive modelling of copper adsorption from aqueous solution on Spirulina platensis. J Hazard Mater 173:123–129. https://doi.org/10.1016/j.jhazmat.2009.08.057
Jiang L, Ye Q, Chen J et al (2018) Preparation of magnetically recoverable bentonite–Fe3O4–MnO2 composite particles for Cd(II) removal from aqueous solutions. J Colloid Interface Sci 513:748–759. https://doi.org/10.1016/j.jcis.2017.11.063
Tzu TW (2013) Sorption of Pb(II), Cd(II), and Ni(II) toxic metal ions by alginate-bentonite. J Environ Prot 04:51–55. https://doi.org/10.4236/jep.2013.41B010
Gopalakannan V, Periyasamy S, Viswanathan N (2016) Synthesis of assorted metal ions anchored alginate bentonite biocomposites for Cr(VI) sorption. Carbohydr Polym 151:1100–1109. https://doi.org/10.1016/j.carbpol.2016.06.030
Mittal A, Ahmad R, Hasan I (2016) Biosorption of Pb2+, Ni2+ and Cu2+ ions from aqueous solutions by l-cystein-modified montmorillonite-immobilized alginate nanocomposite. Desalination Water Treat 57:17790–17807. https://doi.org/10.1080/19443994.2015.1086900
Xia M, Zheng X, Du M et al (2018) The adsorption of Cs+ from wastewater using lithium-modified montmorillonite caged in calcium alginate beads. Chemosphere 203:271–280. https://doi.org/10.1016/j.chemosphere.2018.03.129
Hassan AF, Abdel-Mohsen AM, Fouda MMG (2014) Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr Polym 102:192–198. https://doi.org/10.1016/j.carbpol.2013.10.104
Biosorption of methylene blue from aqueous solution by softstem bulrush (Scirpus tabernaemontani Gmel.) - Li - 2008 - Journal of Chemical Technology & Biotechnology - Wiley Online Library. https://doi.org/10.1002/jctb.1989. Accessed 18 Mar 2020
Ahmad R, Mirza A (2015) Sequestration of heavy metal ions by Methionine modified bentonite/Alginate (Meth-bent/Alg): A bionanocomposite
Fabryanty R, Valencia C, Soetaredjo FE et al (2017) Removal of crystal violet dye by adsorption using bentonite – alginate composite. J Environ Chem Eng 5:5677–5687. https://doi.org/10.1016/j.jece.2017.10.057
Benli B, Boylu F, Can MF et al (2011) Rheological, electrokinetic, and morphological characterization of alginate–bentonite biocomposites. J Appl Polym Sci 122:19–28. https://doi.org/10.1002/app.33627
Chen L, Huang Y, Huang L et al (2011) Characterization of Co(II) removal from aqueous solution using bentonite/iron oxide magnetic composites. J Radioanal Nucl Chem 290:675–684. https://doi.org/10.1007/s10967-011-1337-y
Shabani E, Salimi F, Jahangiri A (2019) Removal of arsenic and copper from water solution using magnetic iron/bentonite nanoparticles (Fe3O4/bentonite). Silicon 11:961–971. https://doi.org/10.1007/s12633-018-9895-z
Wan D, Li W, Wang G et al (2015) Adsorption and heterogeneous degradation of rhodamine B on the surface of magnetic bentonite material. Appl Surf Sci 349:988–996. https://doi.org/10.1016/j.apsusc.2015.05.004
Dao HV, Ngeh LN, Bigger SW, Orbell JD (2006) Achievement of 100% removal of oil from feathers employing magnetic particle technology. J Environ Eng 132:555–559. https://doi.org/10.1061/(ASCE)0733-9372(2006)132:5(555)
Lim S-F, Zheng Y-M, Zou S-W, Chen JP (2008) Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study. Environ Sci Technol 42:2551–2556. https://doi.org/10.1021/es7021889
Zhang H, Liang X, Yang C et al (2016) Nano γ-Fe2O3/bentonite magnetic composites: synthesis, characterization and application as adsorbents. J Alloys Compd 688:1019–1027. https://doi.org/10.1016/j.jallcom.2016.07.036
Eren E, Afsin B (2008) Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated bentonite surfaces. Dyes Pigments 76:220–225. https://doi.org/10.1016/j.dyepig.2006.08.019
Yang S, Okada N, Nagatsu M (2016) The highly effective removal of Cs+ by low turbidity chitosan-grafted magnetic bentonite. J Hazard Mater 301:8–16. https://doi.org/10.1016/j.jhazmat.2015.08.033
Alfaro-Cuevas-Villanueva R, Hidalgo-Vázquez AR, de Cortés Penagos C (2014) Thermodynamic, kinetic, and equilibrium parameters for the removal of lead and cadmium from aqueous solutions with calcium alginate beads. ScientificWorldJournal 2014:647512. https://doi.org/10.1155/2014/647512
Barreca S, Orecchio S, Pace A (2014) The effect of montmorillonite clay in alginate gel beads for polychlorinated biphenyl adsorption: isothermal and kinetic studies. Appl Clay Sci 99:220–228. https://doi.org/10.1016/j.clay.2014.06.037
Tayyebi A, Khanchi A, Ghofrani MB, Outokesh M (2010) Synthesis and characterization of a bentonite-alginate microspherical adsorbent for removal of uranyl ions from aqueous solutions. Sep Sci Technol 45:288–298. https://doi.org/10.1080/01496390903255903
Kapoor A, Viraraghavan T (1998) Use of immobilized bentonite in removal of heavy metals from wastewater. J Environ Eng 124:1020–1024. https://doi.org/10.1061/(ASCE)0733-9372(1998)124:10(1020)
Zou C, Jiang W, Liang J et al (2019) Removal of Pb(II) from aqueous solutions by adsorption on magnetic bentonite. Environ Sci Pollut Res 26:1315–1322. https://doi.org/10.1007/s11356-018-3652-0
Tzu TW, Tsuritani T, Sato K (2013) Sorption of Pb(II), Cd(II), and Ni(II) toxic metal ions by alginate-bentonite. J Environ Prot 4:51–55. https://doi.org/10.4236/jep.2013.41B010
Edathil AA, Pal P, Banat F (2018) Alginate clay hybrid composite adsorbents for the reclamation of industrial lean methyldiethanolamine solutions. Appl Clay Sci 156:213–223. https://doi.org/10.1016/j.clay.2018.02.015
Ai L, Li M, Li L (2011) Adsorption of methylene blue from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: kinetics, isotherms, and thermodynamics. J Chem Eng Data 56:3475–3483. https://doi.org/10.1021/je200536h
Bée A, Talbot D, Abramson S, Dupuis V (2011) Magnetic alginate beads for Pb(II) ions removal from wastewater. J Colloid Interface Sci 362:486–492. https://doi.org/10.1016/j.jcis.2011.06.036
Shukla A, Zhang Y-H, Dubey P et al (2002) The role of sawdust in the removal of unwanted materials from water. J Hazard Mater 95:137–152. https://doi.org/10.1016/S0304-3894(02)00089-4
Olu-Owolabi BI, Popoola DB, Unuabonah EI (2010) Removal of Cu2+ and Cd2+ from aqueous solution by bentonite clay modified with binary mixture of goethite and humic acid. Water Air Soil Pollut 211:459–474. https://doi.org/10.1007/s11270-009-0315-2
Çetinkaya Dönmez G, Aksu Z, Öztürk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34:885–892. https://doi.org/10.1016/S0032-9592(99)00005-9
Chen Y-G, Ye W-M, Yang X-M et al (2011) Effect of contact time, pH, and ionic strength on Cd(II) adsorption from aqueous solution onto bentonite from Gaomiaozi, China. Environ Earth Sci 64:329–336. https://doi.org/10.1007/s12665-010-0850-6
Ijagbemi CO, Baek M-H, Kim D-S (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546. https://doi.org/10.1016/j.jhazmat.2008.11.085
Kumar Reddy DH, Lee SM (2012) Water pollution and treatment technologies. J Environ Anal Toxicol. https://doi.org/10.4172/2161-0525.1000e103
Hsu Y-C, Chiang C-C, Yu M-F (1997) Adsorption behavior of basic dyes on activated clay. Sep Sci Technol 32:2513–2534. https://doi.org/10.1080/01496399708000783
Oubagaranadin JUK, Murthy ZVP, Mallapur VP (2010) Removal of Cu(II) and Zn(II) from industrial wastewater by acid-activated montmorillonite-illite type of clay. Comptes Rendus Chim 13:1359–1363. https://doi.org/10.1016/j.crci.2010.05.024
Wang M, Huang Z-H, Liu G, Kang F (2011) Adsorption of dimethyl sulfide from aqueous solution by a cost-effective bamboo charcoal. J Hazard Mater 190:1009–1015. https://doi.org/10.1016/j.jhazmat.2011.04.041
Djebri N, Boutahala M, Chelali N-E et al (2016) Enhanced removal of cationic dye by calcium alginate/organobentonite beads: Modeling, kinetics, equilibriums, thermodynamic and reusability studies. Int J Biol Macromol 92:1277–1287. https://doi.org/10.1016/j.ijbiomac.2016.08.013
Shawky HA (2011) Improvement of water quality using alginate/montmorillonite composite beads. J Appl Polym Sci 119:2371–2378. https://doi.org/10.1002/app.32694
Munagapati VS, Kim D-S (2017) Equilibrium isotherms, kinetics, and thermodynamics studies for congo red adsorption using calcium alginate beads impregnated with nano-goethite. Ecotoxicol Environ Saf 141:226–234. https://doi.org/10.1016/j.ecoenv.2017.03.036
Kumar NS, Woo H-S, Min K (2012) Equilibrium and kinetic studies on biosorption of 2,4,6-trichlorophenol from aqueous solutions by Acacia leucocephala bark. Colloids Surf B 94:125–132. https://doi.org/10.1016/j.colsurfb.2012.01.048
Futalan CM, Kan C-C, Dalida ML et al (2011) Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr Polym 83:528–536. https://doi.org/10.1016/j.carbpol.2010.08.013
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yari, M., Derakhshi, P., Tahvildari, K. et al. Preparation and Characterization of Magnetic Iron Nanoparticles on Alginate/Bentonite Substrate for the Adsorptive Removal of Pb2+ Ions to Protect the Environment. J Polym Environ 29, 2185–2199 (2021). https://doi.org/10.1007/s10924-020-02028-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-02028-8