Abstract
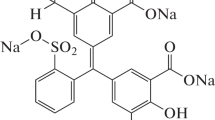
In this research, a simple oxidation chemical process was applied for the synthesis of novel PANI@ZnO nanocomposite. The prepared nanocomposites were characterized by XPS, XRD, FTIR, SEM, TGA and N2 adsorption–desorption isotherms. Thereby, PANI@ZnO highest SBET values (about 40.84 m2 g−1), total mesoporous volume (about 3.214 cm3 g−1) and average pore size (about 46.12 nm). Afterwards, the prepared nanomaterial was applied as novel nanoadsorbent for the adsorption of Congo Red and Methylene Blue dyes from aqueous solutions at 298 K and pH 5.0. Besides, the pseudo-second-order model was obtained the best for the adsorption of both dyes. In the case of isotherm models, the Freundlich model showed the best fit. After removal, the spent adsorbent was regenerated. With the regeneration repeated five cycles, the PANI@ZnO regeneration efficiency remained at a very adequate level. These results are heartening in respect with the objective to utilize them in the field of sensors technology and research related to the photoluminescence sensor application.







Similar content being viewed by others
References
R. Pandimurugan, S. Thambidurai, Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye. J. Environ. Chem. Eng. 4, 1332–1347 (2016)
D.S. Brookstein, Factors associated with textile pattern dermatitis caused by contact allergy to dyes finishes, foam and preservatives. Dermatol. Clin. 27, 309–322 (2009)
P.A. Carneiro, G.A. Umbuzeiro, D.P. Oliveira, M.V.B. Zanoni, Assessment of water contamination caused by a mutagenic textile effluent/dye house effluent bearing disperse dyes. J. Hazard. Mater. 174, 694–699 (2010)
P. Mokhtari, M. Ghaedi, K. Dashtian, M.R. Rahimi, M.K. Purkait, Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: kinetic and isotherm investigation. J. Mol. Liq. 219, 299–305 (2016)
G. Sharma, A. Kumar, M. Naushad, A.G. Peñas, A.H. Al-Muhtaseb, A.A. Ghfar, V. Sharma, T. Ahamad, F.J. Stadler, Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohyd. Polym. 202, 444–453 (2018)
G. Sharma, A. Kumar, S. Sharma, A.H. Al-Muhtaseb, M. Naushad, A.A. Ghfar, T. Ahamad, F.J. Stadler, Fabrication and characterization of novel Fe0@Guar gum-crosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Sep. Purif. Technol. 211, 895–908 (2019)
G. Sharma, D.D. Dionysiou, S. Sharma, A. Kumar, A.H. Al-Muhtaseb, M. Naushad, F.J. Stadler, Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal. Today 335, 437–451 (2019)
A. Paz, J. Carballo, M.J. Perez, J.M. Dominguez, Biological treatment of model dyes and textile wastewater. Chemosphere 181, 168–177 (2017)
S. Sachdeva, A. Kumar, Preparation of nanoporous composite carbon membrane for separation of rhodamine B dye. J. Membr. Sci. 329, 2–10 (2009)
K. Dai, H. Chen, T. Peng, D. Ke, H. Yi, Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous Titania nanoparticles. Chemosphere 69, 1361–1367 (2007)
A. Asfaram, M. Ghaedi, S. Hajati, M. Rezaeinejad, A. Goudarzi, M.K. Purkait, Rapid removal of Auramine-O and Methylene blue by ZnS: Cu nanoparticles loaded on activated carbon: a response surface methodology approach. J. Taiwan Inst. Chem. Eng. 53, 80–91 (2015)
G. Crini, Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. 97, 1061–1085 (2006)
G. Sharma, D. Pathania, M. Naushad, Preparation, characterization, and ion exchange behavior of nanocomposite polyaniline zirconium (IV) selenotungstophosphate for the separation of toxic metal ions. Ionics 21, 1045–1055 (2015)
C. Chen, T. Xu, A. Chen, L. Lu, Y. Gao, Electrosynthesis of poly(N-methylthionine)/polyaniline nanocomposites with enhanced electrochemical and electrocatalytic activities. J. Electrochem. Soc. 163, G159–G165 (2016)
A. Eftekhari, Nanostructured Conductive Polymers (Wiley, New York, 2011)
G. Sharma, D. Pathania, M. Naushad, N.C. Kothiyal, Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: efficient removal of toxic metal ions from water. Chem. Eng. J. 251, 413–442 (2014)
S. Daikh, F.Z. Zeggai, A. Bellil, A. Benyoucef, Chemical polymerization, characterization and electrochemical studies of PANI/ZnO doped with hydrochloric acid and/or zinc chloride: differences between the synthesized nanocomposites. J. Phys. Chem. Solids 121, 78–84 (2018)
D.S. Torres, F. Montilla, F. Huerta, E. Morallón, All electrochemical synthesis of polyaniline/silica sol–gel materials. Electrochim. Acta 56, 3620–3625 (2011)
L. Maaza, F. Djafri, A. Belmokhtar, A. Benyoucef, Evaluation of the influence of Al2O3 nanoparticles on the thermal stability and optical and electrochemical properties of PANI-derived matrix reinforced conducting polymer composites. J. Phys. Chem. Solids 152, 109970 (2021)
M.A. Bekhti, M.S. Belardja, M. Lafjah, F. Chouli, A. Benyoucef, Enhanced tailored of thermal stability, optical and electrochemical properties of PANI matrix containing Al2O3 hybrid materials synthesized through in situ polymerization. Polym. Compos. 42, 6–14 (2021)
M.S. Belardja, H. Djelad, M. Lafjah, F. Chouli, A. Benyoucef, The influence of the addition of tungsten trioxide nanoparticle size on structure, thermal, and electroactivity properties of hybrid material-reinforced PANI. Colloid Polym. Sci. 298, 1455–1463 (2020)
L. Hongjun, Z. Zang, X. Tang, Synthesis mechanism and optical properties of well nanoflower-shaped ZnO fabricated by a facile method. Optical Mater. Express. 4, 1762–1769 (2014)
R. Viter, M. Savchuk, N. Starodub, Z. Balevicius, S. Tumenas, A. Ramanaviciene, D. Jevdokimovs, D. Erts, I. IgorIatsunskyi, A. Ramanavicius, Photoluminescence immunosensor based on bovine leukemia virus proteins immobilized on the ZnO nanorods. Sens. Actuators B Chem. 285, 601–606 (2019)
Z. Balevicius, A. Paulauskas, I. Plikusiene, L. Mikoliunaite, M. Bechelany, A. Popov, A. Ramanavicius, A. Ramanaviciene, Towards application of Al2O3/ZnO nanolaminates in immunosensors: total internal reflection spectroscopic ellipsometry based evaluation of BSA immobilization. J. Mater. Chem. C. 6, 8778–8783 (2018)
R. Viter, K. Kunene, P. Genys, D. Jevdokimovs, D. Erts, A. Sutka, K. Bisetty, A. Viksna, A. Ramanaviciene, A. Ramanavicius, Photoelectrochemical bisphenol S sensor based on ZnO nanoroads modified by molecularly imprinted polypyrrole. Macromol. Chem. Phys. 221, 1900232 (2020)
M. Turemis, D. Zappi, M.T. Giard, G. Basile, A. Ramanaviciene, A. Kapralovs, A. Ramanavicius, R. Viter, ZnO/polyaniline composite based photoluminescence sensor for the determination of acetic acid vapor. Talanta 211, 120658 (2020)
V. Eskizeybek, F. Sarı, H. Gülce, A. Gülce, A. Avcı, Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun light irradiations. Appl. Catal. B 119–120, 197–206 (2012)
O. Mahi, K. Khaldi, M.S. Belardja, A. Belmokhtar, A. Benyoucef, Development of a new hybrid adsorbent from Opuntia Ficus Indica NaOH activated with PANI reinforced and its potential use in orange G dye removal. J. Inorg. Organomet. Polym Mater. 31, 2095–2104 (2021)
R. Kumar, S.A. Ansari, M.A. Barakat, A. Aljaafari, M.H. Cho, A polyaniline@MoS2-based organic–inorganic nanohybrid for the removal of Congo red: adsorption kinetic, thermodynamic and isotherm studies. N. J. Chem. 40, 18802–18809 (2018)
R. Ahmad, R. Kumar, Conducting polyaniline/iron oxide composite: a novel adsorbent for the removal of amido black 10B. J. Chem. Eng. Data 55, 3489–3493 (2010)
F.Z. Kouidri, I. Moulefera, S. Bahoussi, A. Belmokhtar, A. Benyoucef, Development of hybrid materials based on carbon black reinforced poly(2-methoxyaniline): preparation, characterization and tailoring optical, thermal and electrochemical properties. Colloid Polym. Sci. (2021). https://doi.org/10.1007/s00396-021-04837-2
A. Belalia, A. Zehhaf, A. Benyoucef, Preparation of hybrid material based of PANI with SiO2 and its adsorption of phenol from aqueous solution. Polym. Sci., Ser. B 60, 816–824 (2018)
M. Zenasni, A.Q. Jaime, A. Benyoucef, A. Benghalem, Synthesis and characterization of polymer/V2O5 composites based on poly(2-aminodiphenylamine). Polym. Compos. 42, 1064–1074 (2021)
M.H. Sadeghi, M.A. Tofighy, T. Mohammadi, One-dimensional graphene for efficient aqueous heavy metal adsorption: rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 253, 126647 (2020)
A.B. Wozniak, R. Pietrzak, Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. Chem. Eng. J. 393, 124785 (2020)
R.R. Amador, J. Alvarado, G.F. Carrasco, L.M. de la Garza, S.A. Iniesta, A.L. Flores, Y.P. Bernal, M.A.M. Rojas, J.J.G. Arciniega, H.P.M. Hernández, J.F.C. Vega, J.B. Camacho, The influence of deposition time on the structural, morphological, optical and electrical properties of ZnO-rGO nanocomposite thin films grown in a single step by USP. Curr. Comput. Aided Drug Des. 10, 1–21 (2020)
M. Javed, S.M. Abbas, M. Siddiq, D. Han, L. Niu, Mesoporous silica wrapped with graphene oxide-conducting PANI nanowires as a novel hybrid electrode for supercapacitor. J. Phys. Chem. Solids 113, 220–228 (2018)
Z. Yang, Q.H. Liu, The structural and optical properties of ZnO nanorods via citric acid-assisted annealing route. J. Mater. Sci. 43, 6527–6530 (2008)
G. Sharma, B. Thakur, A. Kumar, S. Sharma, M. Naushad, F.J. Stadler, Atrazine removal using chitin-cl-poly(acrylamide-co-itaconic acid) nanohydrogel: isotherms and pH responsive nature. Carbohydr. Polymers. 241, 116258 (2020)
S. Matindoust, A. Farzi, M.B. Nejad, M.H.S. Abadi, Z. Zou, L.R. Zheng, Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 28, 7760–7768 (2017)
V. Gilja, I. Vrban, V. Mandić, M. Žic, Z.H. Murgić, Preparation of a PANI/ZnO composite for efficient photocatalytic degradation of acid blue. Polymers 10, 1–17 (2018)
C. Bao, M. Chen, X. Jin, D. Hu, Q. Huang, Efficient and stable photocatalytic reduction of aqueous hexavalent chromium ions by polyaniline surface-hybridized ZnO nanosheets. J. Mol. Liq. 279, 133–145 (2019)
G. Sriram, U. Uthappa, D. Losic, M. Kigga, H.Y. Jung, M.D. Kurkuri, Mg–Al-layered double hydroxide (LDH) modified diatoms for highly efficient removal of Congo red from aqueous solution. Appl. Sci. 10, 2285 (2020)
T. Lindfors, A. Ivaska, pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 531, 43–52 (2002)
I. Plikusiene, Z. Balevicius, A. Ramanaviciene, J. Talbot, G. Mickiene, S. Balevicius, A. Stirke, A. Tereshchenko, L. Tamosaitis, G. Zvirblis, A. Ramanavicius, Evaluation of affinity sensor response kinetics towards dimeric ligands linked with spacers of different rigidity: Immobilized recombinant granulocyte colony-stimulating factor based synthetic receptor binding with genetically engineered dimeric analyte derivatives. Biosensors Bioelectron. 156, 112112 (2020)
H. Zhang, J. Ma, F. Wang, Y. Chu, L. Yang, M. Xia, Mechanism of carboxymethyl chitosan hybrid montmorillonite and adsorption of Pb(II) and Congo red by CMC-MMT organic-inorganic hybrid composite. Int. J. Biol. Macromol. 149, 1161–1169 (2020)
A.O. Adesina, O.A. Elvis, N.D.S. Mohallem, S.J. Olusegun, Adsorption of Methylene blue and Congo red from aqueous solution using synthesized alumina–zirconia composite. Environ. Technol. 42, 1061–1070 (2021)
C.M. Simonescu, A. Tătăruş, D.C. Culiţă, N. Stănică, I.A. Ionescu, B. Butoi, A.M. Banici, Comparative study of CoFe2O4 nanoparticles and CoFe2O4-chitosan composite for Congo red and methyl orange removal by adsorption. Nanomaterials 11, 1–24 (2021)
R. Wo, Q.L. Li, C. Zhu, Y. Zhang, G.F. Qiao, K.Y. Lei, P. Du, W. Jiang, Preparation and characterization of functionalized metal-organic frameworks with core/shell magnetic particles (Fe3O4@SiO2@MOFs) for removal of Congo red and methylene blue from water solution. J. Chem. Eng. Data 64, 2455–2463 (2019)
C. Aoopngan, J. Nonkumwong, S. Phumying, W. Promjantuek, S. Maensiri, P. Noisa, S. Pinitsoontorn, S. Ananta, L. Srisombat, Amine-functionalized and hydroxyl-functionalized magnesium ferrite nanoparticles for Congo red adsorption. ACS Appl. Nano Mater. 2, 5329–5341 (2019)
H. Chafai, M. Laabd, S. Elbariji, M. Bazzaoui, A. Albourine, Study of Congo red adsorption on the polyaniline and polypyrrole. J. Dispers. Sci. Technol. 38, 832–836 (2017)
M.O. Ansari, R. Kumar, S.A. Ansari, S.P. Ansari, M.A. Barakat, A. Alshahrie, M.H. Cho, Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composite for Cr(VI) and Congo red adsorption. J. Colloid Interface Sci. 496, 407–415 (2017)
R. Kumar, M.O. Ansari, N. Parveen, M.A. Barakat, M.H. Cho, Simple route for the generation of differently functionalized PVC@graphene-polyaniline fiber bundles for the removal of Congo red from wastewater. RSC Adv. 5, 61486–61494 (2015)
S. Singh, S. Perween, A. Ranjan, Dramatic enhancement in adsorption of Congo red dye in polymer-nanoparticle composite of polyaniline-zinc titanate. J. Environ. Chem. Eng. 9, 105149 (2021)
S. Dhananasekaran, R. Palanivel, S. Pappu, Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by α-chitin nanoparticles. J. Adv. Res. 7, 113–124 (2016)
D.S. Franco, E.H. Tanabe, D.A. Bertuol, G.S. Dos Reis, E.C. Lima, G.L. Dotto, Alternative treatments to improve the potential of rice husk as adsorbent for methylene blue. Water Sci Technol 75, 296–305 (2017)
M.O. Bello, N. Abdus-Salam, F.A. Adekola, U. Pal, Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem. Data Collect. 31, 100607 (2021)
R. Jamal, L. Zhang, M. Wang, Q. Zhao, T. Abdiryim, Synthesis of poly(3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Progress Nat. Sci. Mater. Int. 26, 32–40 (2016)
M.M. Ayad, A.A. EI-Nasr, Adsorption of cationic dye (Methylene Blue) from water using polyaniline nanotubes base. J. Phys. Chem. C. 114, 14377–14383 (2010)
M. Ayad, S. Zaghlol, Nanostructured crosslinked polyaniline with high surface area: synthesis characterization and adsorption for organic dye. Chem. Eng. J. 204–206, 79–86 (2012)
Acknowledgements
Authors gratefully acknowledge the Algerian Ministry of Higher Education and Scientific Research, and also University Materials Science Institute of Alicante Spain for the co-operation availing.
Funding
There is no financial sources funding regarding of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toumi, I., Djelad, H., Chouli, F. et al. Synthesis of PANI@ZnO Hybrid Material and Evaluations in Adsorption of Congo Red and Methylene Blue Dyes: Structural Characterization and Adsorption Performance. J Inorg Organomet Polym 32, 112–121 (2022). https://doi.org/10.1007/s10904-021-02084-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02084-0